High Resolution Analysis of Tryptic Digests of Bovine Serum Albumin using UHPLC with Photodiode Array Detection
January 5, 2024
Introduction
Peptide mapping is a standard testing method for biomedicines. This method requires HPLC separation of peptide segments digested using enzymes or chemicals and is an internationally harmonized method described by the U.S. Pharmacopoeia (USP), the European Pharmacopoeia (EP)and the Japanese Pharmacopoeia (JP).
The target protein and a standard protein are digested using the same protocol. A direct comparison of the peptide map of the target protein can be made with the standard protein so that the expression of recombinant proteins can be assessed. This method is applied to the quality assurance of a variety of food products.

Experimental
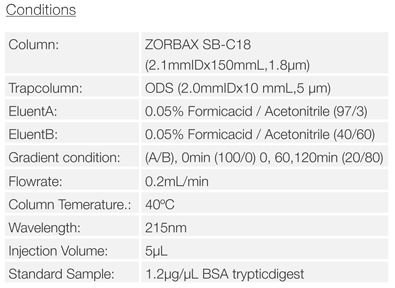
Sample Preparation
- Dissolve 10 mg of BSA in 1.5 mL of solvent (6 M Urea: 0.1M NH4HCO3=1:4).
- Add 200 μl of 1% of trypsin in 0.003N HClto BSA solution to a ratio of BSA solution to 1% trypsin at 5:1 (w/w).
- Digest and incubate at 37ºC for 15 hours.
- Perform ultrafiltration using model Ultrafree C3 UFC3LGC00 (10,000MWCO) membrane.
- Dilute5 times with mobile phase A.
Keywords
440013H
Results
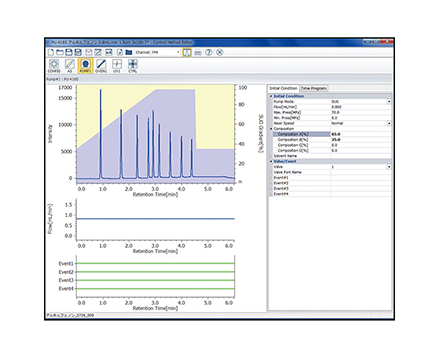
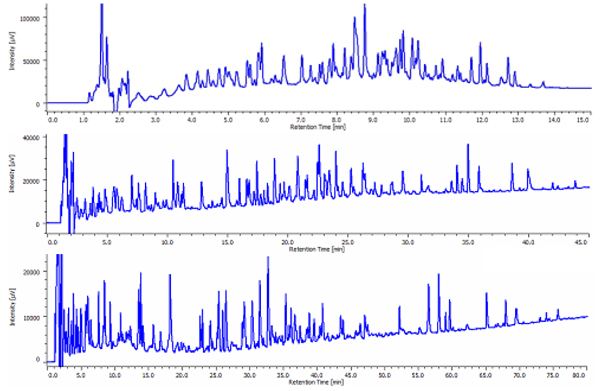
Chromatograms of tryptic digests are shown in figure 1. Depending on the gradient profiles, the number of eluted peak varies: 93 peaks in a 10 minute gradient,139 peaks in a 60 minute gradient and 125 peaks in a 120 minute gradient.
Featured Products:

High Resolution Analysis of Tryptic Digests of Bovine Serum Albumin using UHPLC with Photodiode Array Detection
Introduction
Peptide mapping is a standard testing method for biomedicines. This method requires HPLC separation of peptide segments digested using enzymes or chemicals and is an internationally harmonized method described by the U.S. Pharmacopoeia (USP), the European Pharmacopoeia (EP)and the Japanese Pharmacopoeia (JP).
The target protein and a standard protein are digested using the same protocol. A direct comparison of the peptide map of the target protein can be made with the standard protein so that the expression of recombinant proteins can be assessed. This method is applied to the quality assurance of a variety of food products.

Experimental


Sample Preparation
- Dissolve 10 mg of BSA in 1.5 mL of solvent (6 M Urea: 0.1M NH4HCO3=1:4).
- Add 200 μl of 1% of trypsin in 0.003N HClto BSA solution to a ratio of BSA solution to 1% trypsin at 5:1 (w/w).
- Digest and incubate at 37ºC for 15 hours.
- Perform ultrafiltration using model Ultrafree C3 UFC3LGC00 (10,000MWCO) membrane.
- Dilute5 times with mobile phase A.
Results
Chromatograms of tryptic digests are shown in figure 1. Depending on the gradient profiles, the number of eluted peak varies: 93 peaks in a 10 minute gradient,139 peaks in a 60 minute gradient and 125 peaks in a 120 minute gradient.

Keywords
440013H

 Download This Application
Download This Application