High-Throughput Circular Dichroism for the Analysis of Biomedicines and pH Dependency
January 5, 2024
Introduction
High Throughput Circular Dichroism Measurement of Biomedicines
High throughput circular dichroism measurement can be important for the evaluation of large numbers of samples. In this application note, we illustrate the use of the automated HTCD system to evaluate the pH dependency of the structure of human serum albumin (HSA).
Biomedicines offer a more natural approach to medicinal treatment. Biomedicines often include active ingredients derived from proteins, and the R&D into these materials is increasing rapidly. However, biomedicines are more sensitive to environmental changes, such as a change in temperature, pH, and salt concentration, compared with more traditional small-molecule pharmaceuticals. This environmental sensitivity may be a potential cause of biomedicines’ deactivation during manufacture and storage.
Circular dichroism (CD) measurements can provide information regarding changes in protein structure in small quantities of sample. Since protein structure and activity are closely related, CD measurements are now widely accepted in the quality control of protein, which includes biomedicines.
To meet the demand for increased sample throughput in the modern pharmaceutical laboratory, JASCO has developed a fully automated high throughput circular dichroism system. This system is composed of a J-1500 Circular Dichroism spectrophotometer with an automatic sample handling system for use with microplates and sample tubes. The high-throughput circular dichroism system enables the automation of sample pretreatment, measurement, and flow cell cleaning (to minimize carry-over).
Experimental
The pH of human serum albumin (reagent 1) was adjusted by diluted sulfuric acid or sodium hydroxide (reagent 2) using a 1:4 ratio. The initial concentration of the 30 mg of HSA used was 0.05 mg/mL and the final concentration after mixing was 0.01 mg/mL. The mixed reagent was injected into a 10 mm rectangular cell in the sample compartment of the J-1500. The entire sampling procedure, including the mixing of reagents, CD spectral measurement, and the washing and drying of cells were pre-programmed so that a fully automated and unattended measurement could be performed.
| Measurement Conditions | |
|---|---|
| Data Acquisition Interval | 0.5 nm |
| Path Length | 10 mm |
| Spectral Bandwidth | 1 nm |
| Scan Speed | 100 nm/min |
| Accumulations | 2 |
| Response Time | 1 sec |
| Reagent 1 Concentration (HSA) | 0.5 mg/mL |
| Reagent 1 Used | 30 mg |
| Mixed Reagent Concentration | 0.01 mg/mL |
Keywords
200-CD-0021, Biomedicines, quality control, automated measurement, high-throughput screening, human serum albumin, circular dichroism, J-1500, ASU-800, biochemistry, pharmaceutical
Results
Figure 1 shows the CD spectra of human serum albumin for 10 different pH values (1.3, 2.2, 3.1, 4.1, 5.4, 6.7, 7.5, 8.4, 9.3, 10.7). The plot illustrates that the CD decreases as the pH increases, indicating structural changes to HSA.
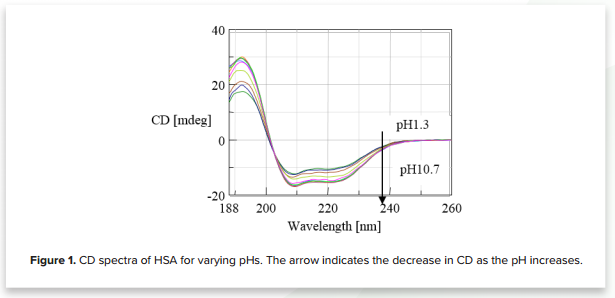
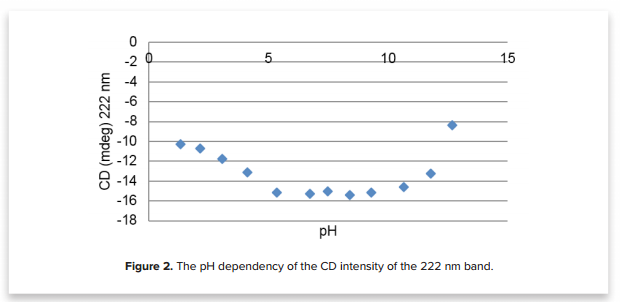
Figure 2 shows these structural changes in more detail by plotting the CD values at 222 nm as a function of pH. 222 nm is a CD marker band for α-helices in proteins. By plotting this band, we can show the structural deviation of the protein’s α-helical content. Between pH 5 to 10, the α-helical structure is conserved. However, in acidic conditions (<5) and basic conditions (>10), the decreased CD intensity suggests a slight denaturation of the HSA protein.
Conclusion
By monitoring the pH dependency of human serum albumin using the J-1500 Circular Dichroism spectrometer and sample-handling ASU-800, numerous samples can be automatically measured to determine the structural integrity of the protein. This application note demonstrates that CD measurement is an effective tool for the quality control of biomedicines and that the JASCO J-1500 high-throughput CD system can assist pharmaceutical laboratories in the unattended screening of large numbers of samples.
Featured Products:
-
High-Throughput CD
-

Highest performance with a wide range of accessories for maximum flexibility to meet complex research demands.
J-1500
-
J-1700

High-Throughput Circular Dichroism for the Analysis of Biomedicines and pH Dependency
Introduction
High Throughput Circular Dichroism Measurement of Biomedicines
High throughput circular dichroism measurement can be important for the evaluation of large numbers of samples. In this application note, we illustrate the use of the automated HTCD system to evaluate the pH dependency of the structure of human serum albumin (HSA).
Biomedicines offer a more natural approach to medicinal treatment. Biomedicines often include active ingredients derived from proteins, and the R&D into these materials is increasing rapidly. However, biomedicines are more sensitive to environmental changes, such as a change in temperature, pH, and salt concentration, compared with more traditional small-molecule pharmaceuticals. This environmental sensitivity may be a potential cause of biomedicines’ deactivation during manufacture and storage.
Circular dichroism (CD) measurements can provide information regarding changes in protein structure in small quantities of sample. Since protein structure and activity are closely related, CD measurements are now widely accepted in the quality control of protein, which includes biomedicines.
To meet the demand for increased sample throughput in the modern pharmaceutical laboratory, JASCO has developed a fully automated high throughput circular dichroism system. This system is composed of a J-1500 Circular Dichroism spectrophotometer with an automatic sample handling system for use with microplates and sample tubes. The high-throughput circular dichroism system enables the automation of sample pretreatment, measurement, and flow cell cleaning (to minimize carry-over).
Experimental
The pH of human serum albumin (reagent 1) was adjusted by diluted sulfuric acid or sodium hydroxide (reagent 2) using a 1:4 ratio. The initial concentration of the 30 mg of HSA used was 0.05 mg/mL and the final concentration after mixing was 0.01 mg/mL. The mixed reagent was injected into a 10 mm rectangular cell in the sample compartment of the J-1500. The entire sampling procedure, including the mixing of reagents, CD spectral measurement, and the washing and drying of cells were pre-programmed so that a fully automated and unattended measurement could be performed.
| Measurement Conditions | |
|---|---|
| Data Acquisition Interval | 0.5 nm |
| Path Length | 10 mm |
| Spectral Bandwidth | 1 nm |
| Scan Speed | 100 nm/min |
| Accumulations | 2 |
| Response Time | 1 sec |
| Reagent 1 Concentration (HSA) | 0.5 mg/mL |
| Reagent 1 Used | 30 mg |
| Mixed Reagent Concentration | 0.01 mg/mL |
Results
Figure 1 shows the CD spectra of human serum albumin for 10 different pH values (1.3, 2.2, 3.1, 4.1, 5.4, 6.7, 7.5, 8.4, 9.3, 10.7). The plot illustrates that the CD decreases as the pH increases, indicating structural changes to HSA.

Figure 2 shows these structural changes in more detail by plotting the CD values at 222 nm as a function of pH. 222 nm is a CD marker band for α-helices in proteins. By plotting this band, we can show the structural deviation of the protein’s α-helical content. Between pH 5 to 10, the α-helical structure is conserved. However, in acidic conditions (<5) and basic conditions (>10), the decreased CD intensity suggests a slight denaturation of the HSA protein.

Conclusion
By monitoring the pH dependency of human serum albumin using the J-1500 Circular Dichroism spectrometer and sample-handling ASU-800, numerous samples can be automatically measured to determine the structural integrity of the protein. This application note demonstrates that CD measurement is an effective tool for the quality control of biomedicines and that the JASCO J-1500 high-throughput CD system can assist pharmaceutical laboratories in the unattended screening of large numbers of samples.
Keywords
200-CD-0021, Biomedicines, quality control, automated measurement, high-throughput screening, human serum albumin, circular dichroism, J-1500, ASU-800, biochemistry, pharmaceutical

 Download This Application
Download This Application

