Rapid Profiling of Prostanoids in Biological Samples using UHPLC
August 25, 2022
Introduction
Inflammation is implicated in number of diseases including, hypertension, ischemic heart injury rheumatoid arthritis and atherosclerosis. Therefore, a mechanistic understanding of the inflammation process at the molecular level would contribute to the mechanism of the disease state, injury and recovery. Eicosanoids are specific biomarkers for inflammation. Arachidonic acid (AA) which is an omega-6 polyunsaturated fatty acid can undergo oxidative metabolism by cyclooxygenase (COX), lipoxygenase (LOX) or cytochrome (P450) to produce a number of these inflammatory lipid biomolecules.
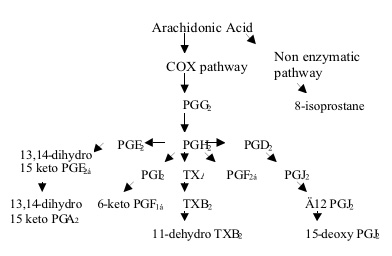
The oxidation of AA by COX-2 results in a number of prostanoids which include PGE2, PGD2 and PGF2, PGJ2, prostacyclin (PGI2) and thromboxane A2 (TXA2). Prostacyclin is considered to be a potent vasodilator whereas thromboxane A2 is considered to be a potent vasoconstrictor. PGI2 and TXA2 are unstable at physiological pH and are readily converted to 6-keto PGF1α and 11-dehydro TXB2 respectively. Hence these are considered to be reliable for the in vivo estimation of PGI2 and TXA2 respectively. PGE2, PGD2 and PGF2 are considered to be pro inflammatory biomarkers.13, 14 -dihydro-15-keto PGE2 and 13, 14 -dihydro-15-keto PGA2 are major metabolites of PGE2 in plasma.15-deoxy PGJ2 which is the major metabolite of PGJ2 is thought to have anti inflammatory properties especially in rheumatoid arthritis. Free radical peroxidation of AA produces a series of prostaglandin like compounds known as the isoprostanes of which F2 isoprostane which are PGF2 like compounds is considered to be the most important.8-iso PGF2 also known as 8-isoprostane the most abundant form of the F2 isoprostane is considered to be an indicator of in vivo oxidative stress Figure 1 shows the pathways elucidated for the metabolism of arachidonic acid.
This application note describes a 6.8 min method for identifying a mixture of 9 prostanoids using UHPLC. Separation of these compounds using conventional HPLC typically takes around 21 minutes.
Experimental
Sample Preparation
8-iso PGF2 was supplied in methanol with a stock concentration of 1 mg/ml and 13, 14 -dihydro-15-keto PGE2 and 13, 14 -dihydro-15-keto PGA2 were supplied in methyl acetate with a stock concentration of 1 mg/ml. All the other prostanoids were dissolved in 1 ml methanol such that the stock concentration for all was 1mg/ml.Working standards for the analytes were prepared by serial dilution using acetonitrile.
X-LC System and Operating Conditions

System: X-LC System
Mobile Phase A: 0.1 % Formic Acid
Mobile Phase B: ACN
Flow Rate: 0.2 ml/min
Gradient:
| Time (min) | B (%) |
|---|---|
| 0.0 | 35 |
| 4.0 | 35 |
| 6.0 | 90 |
| 6.5 | 35 |
| 6.8 | 35 |
Injection volume
2µl
Stationary Phase
Column: Restek Pinnacle DB C18 1.9µm (50*2.1 mm)
Column Temperature: 25°C
UV Detection
| Time (min) | Wavelength (nm) |
|---|---|
| 0.0 | 220 |
| 1.0 | 196 |
Results
Table 1 provides a list of the compounds from the method and figure 2 shows a UHPLC chromatogram of a standard mixture of prostanoids (150 ng each). The compounds were separated using a gradient elution of 0.1 % formic acid and acetonitrile on a Restek Pinnacle DB C18 1.9µm (50*2.1 mm) at a flow rate of 0.2 ml/min.
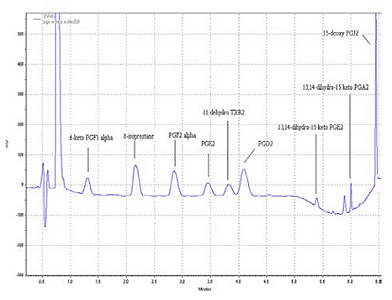
| Compound Name | Symbol | RT (min) |
|---|---|---|
| 6-keto-ProstaglandinF1α | 6-keto PGF1α | 1.25 |
| 8-isoprostane | 8-iso PGF2α | 2.25 |
| Prostaglandin F2α | PGF2α | 2.88 |
| 11-dehydro Thromboxane B2 | 11-dehydro TXB2 | 3.86 |
| Prostaglandin D2 | PGD2 | 4.18 |
| 13,14-dihydro-15 keto Prostaglandin E2 | 13,14-dihydro-15 keto PGE2 | 5.43 |
| 13,14-dihydro-15 keto Prostaglandin A2 | 13,14-dihydro-15 keto PGA2 | 6.01 |
| 15-deoxy Prostaglandin J2 | 15-deoxy PGJ2 | 6.48 |
Featured Products:

Rapid Profiling of Prostanoids in Biological Samples using UHPLC
Introduction
Inflammation is implicated in number of diseases including, hypertension, ischemic heart injury rheumatoid arthritis and atherosclerosis. Therefore, a mechanistic understanding of the inflammation process at the molecular level would contribute to the mechanism of the disease state, injury and recovery. Eicosanoids are specific biomarkers for inflammation. Arachidonic acid (AA) which is an omega-6 polyunsaturated fatty acid can undergo oxidative metabolism by cyclooxygenase (COX), lipoxygenase (LOX) or cytochrome (P450) to produce a number of these inflammatory lipid biomolecules.
The oxidation of AA by COX-2 results in a number of prostanoids which include PGE2, PGD2 and PGF2, PGJ2, prostacyclin (PGI2) and thromboxane A2 (TXA2). Prostacyclin is considered to be a potent vasodilator whereas thromboxane A2 is considered to be a potent vasoconstrictor. PGI2 and TXA2 are unstable at physiological pH and are readily converted to 6-keto PGF1α and 11-dehydro TXB2 respectively. Hence these are considered to be reliable for the in vivo estimation of PGI2 and TXA2 respectively. PGE2, PGD2 and PGF2 are considered to be pro inflammatory biomarkers.13, 14 -dihydro-15-keto PGE2 and 13, 14 -dihydro-15-keto PGA2 are major metabolites of PGE2 in plasma.15-deoxy PGJ2 which is the major metabolite of PGJ2 is thought to have anti inflammatory properties especially in rheumatoid arthritis. Free radical peroxidation of AA produces a series of prostaglandin like compounds known as the isoprostanes of which F2 isoprostane which are PGF2 like compounds is considered to be the most important.8-iso PGF2 also known as 8-isoprostane the most abundant form of the F2 isoprostane is considered to be an indicator of in vivo oxidative stress Figure 1 shows the pathways elucidated for the metabolism of arachidonic acid.
This application note describes a 6.8 min method for identifying a mixture of 9 prostanoids using UHPLC. Separation of these compounds using conventional HPLC typically takes around 21 minutes.
Experimental

Sample Preparation
8-iso PGF2 was supplied in methanol with a stock concentration of 1 mg/ml and 13, 14 -dihydro-15-keto PGE2 and 13, 14 -dihydro-15-keto PGA2 were supplied in methyl acetate with a stock concentration of 1 mg/ml. All the other prostanoids were dissolved in 1 ml methanol such that the stock concentration for all was 1mg/ml.Working standards for the analytes were prepared by serial dilution using acetonitrile.
X-LC System and Operating Conditions

System: X-LC System
Mobile Phase A: 0.1 % Formic Acid
Mobile Phase B: ACN
Flow Rate: 0.2 ml/min
Gradient:
| Time (min) | B (%) |
|---|---|
| 0.0 | 35 |
| 4.0 | 35 |
| 6.0 | 90 |
| 6.5 | 35 |
| 6.8 | 35 |
Injection volume
2µl
Stationary Phase
Column: Restek Pinnacle DB C18 1.9µm (50*2.1 mm)
Column Temperature: 25°C
UV Detection
| Time (min) | Wavelength (nm) |
|---|---|
| 0.0 | 220 |
| 1.0 | 196 |
Results
Table 1 provides a list of the compounds from the method and figure 2 shows a UHPLC chromatogram of a standard mixture of prostanoids (150 ng each). The compounds were separated using a gradient elution of 0.1 % formic acid and acetonitrile on a Restek Pinnacle DB C18 1.9µm (50*2.1 mm) at a flow rate of 0.2 ml/min.

| Compound Name | Symbol | RT (min) |
|---|---|---|
| 6-keto-ProstaglandinF1α | 6-keto PGF1α | 1.25 |
| 8-isoprostane | 8-iso PGF2α | 2.25 |
| Prostaglandin F2α | PGF2α | 2.88 |
| 11-dehydro Thromboxane B2 | 11-dehydro TXB2 | 3.86 |
| Prostaglandin D2 | PGD2 | 4.18 |
| 13,14-dihydro-15 keto Prostaglandin E2 | 13,14-dihydro-15 keto PGE2 | 5.43 |
| 13,14-dihydro-15 keto Prostaglandin A2 | 13,14-dihydro-15 keto PGA2 | 6.01 |
| 15-deoxy Prostaglandin J2 | 15-deoxy PGJ2 | 6.48 |

 Download This Application
Download This Application