What is the Raman Effect?
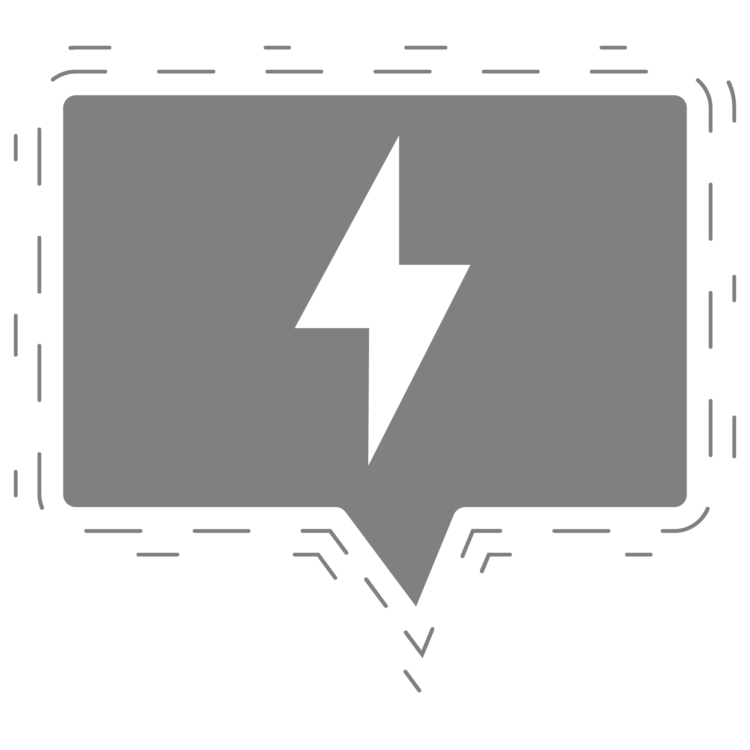
Raman spectroscopy is a popular technique for the analysis of molecular structure and is considered complementary to infrared spectroscopy. Raman spectroscopy is based on the Raman effect which was first identified by physicist Chandrasekhara Venkata Raman in 1928. The Raman effect is based on the scattering of light, which includes both elastic scatter (Rayleigh) that occurs at the same wavelength as the incident light and inelastic scatter (Raman) at different wavelengths, due to molecular bond vibration. Raman scatter is about a million times less intense than Rayleigh scatter. Therefore, to obtain Raman spectra, it is necessary to prevent the Rayleigh scatter overpowering the weaker Raman scatter.
In a typical Raman spectrometer, spectra are measured by exciting the sample by a high intensity laser, with the resulting scattered light being passed to a spectrograph. The Raman shift is the energy difference between the incident light and scattered light. In the resulting spectrum the vertical axis is the intensity of the scattered light and the horizontal axis is the frequency of the Raman shift (cm-1) in the spectrum.
What is the Raman shift?
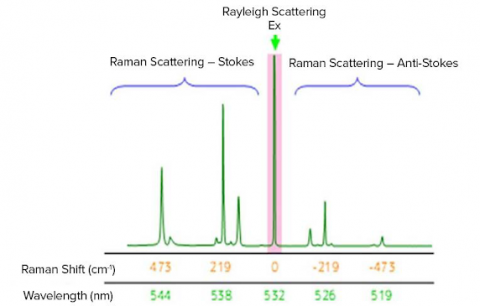
The Raman shift is associated with two different energy bands. The shift at wavelengths higher than that of the incident light is termed Stokes scattering. The shift at wavelengths lower than that of the incident light is termed anti-Stokes scattering. As an example, the Raman spectrum of sulfur measured with an excitation wavelength of 532 nm, green laser(shown in the figure below). Stokes scattering is observed in the lower wavenumber (longer wavelength) region and anti-Stokes scattering in the higher wavenumber (shorter wavelength) region. Typically, higher-intensity Stokes scattering peaks are used for analysis, but anti-Stokes peaks can also be used.
Difference between Raman spectroscopy and IR spectroscopy
Both Raman spectroscopy and IR spectroscopy are based on molecular vibrations as illustrated below. Infrared spectroscopy is based on absorption of light energy corresponding to the vibrational energy of molecules. Raman spectroscopy is based on scattering of incident light at an energy shifted by the vibrational energy (hν) of the molecule. Vibration modes for the same functional groups are observed at the same wavenumber.
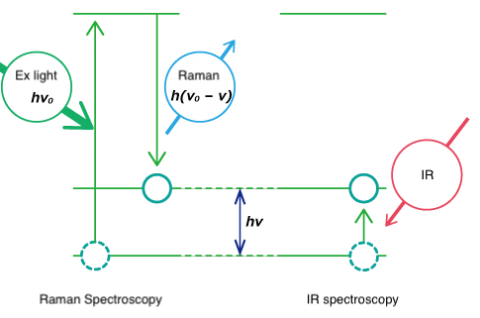
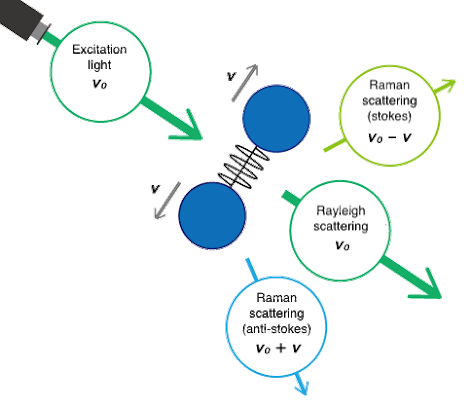
Though both are forms of vibrational spectroscopy, IR and Raman spectroscopy differ in some fundamental aspects, as shown in Fig. 4. IR spectroscopy is based on the fact that molecular absorption at specific vibrational frequencies causes a change in the dipole moment. Raman spectroscopy relies on the change in the polarizability of a molecule at the frequencies (Raman shift) at which the molecule scatters radiation. IR spectroscopy is sensitive to hetero-nuclear functional group vibrations and polar bonds, especially OH stretching in water. Raman spectroscopy is sensitive to homo-nuclear molecular bonds such as C-C, C=C and C≡C bonds.
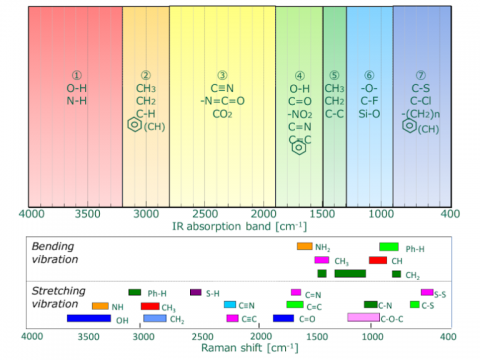
A comparison of IR transmission and Raman spectra for L-cystine is shown in Fig. 5. The intensity of the two spectra exhibit mirror symmetry, so IR and Raman spectra are often considered to be “complementary”. But they are different in the type of physical phenomenon they can measure. In IR measurements, the spectral intensity depends on the size of the dipole moment for vibration modes for bonds such as C=O and O-H. On the other hand, in Raman spectroscopy, the intensity depends on the degree of polarizability (electron volume) for vibration modes for bonds such as S-S, C-C, and CN.