FTIR Microscopy for the Analysis of Polymer Laminates in Beverage Packaging
August 24, 2022
Introduction
Laminates are combinations of polymers that combine the benefits and properties of the various polymers used in the laminate to create a better material. Polymer laminates have a multitude of uses including packaging for food and other products. Laminates act as moisture and/or oxygen barriers and as such are commonly used to protect foods and chemicals. Analysis of laminates is often required for quality control and to determine laminate adhesion problems.
Modern polymers are seldom used as pure materials, more often mixtures of different polymers are combined to give the desired physical characteristics. For example, the rigidity of polystyrene is often combined with an elastic polymer to produce stiff yet shock resistant materials. Polymeric laminates are important in the microelectronics, medical, food packaging, and chemical industries. Since, laminates act as moisture and/or oxygen barriers they are commonly used to protect foods and chemicals.
There are many methods that can be used to generate these mixtures but one of the most common is to simply assemble the polymers into layers and create a laminate. The most widely used structural spectroscopic measurement for laminates is Fourier Transform infrared (FTIR). FTIR spectroscopy is a non-destructive technique that that allows chemical identification of the component materials in the laminate.
Infrared spectroscopy provides the ability to study the interactions of the vibrational and rotational energies of atoms or groups of atoms within molecules. Infrared spectra reflect vibrational motions that produce a change in the permanent dipole moment of the molecule. Infrared spectroscopy is a powerful qualitative and quantitative tool. There is a large amount of information that can be gained by using infrared spectroscopy for the analysis of polymers. Minor changes to the molecular structure usually result in a spectrum that is clearly distinguishable from the spectrum of the original compound. Thus, they can be used to identify the presence of a specific chemical compound or mixture of compounds.
Experimental
Several different polymer materials were selected for analysis including a prepared juice bottle and a plastic food container. Laminate samples of controlled thickness were sectioned with a razor blade and the cross section examined using the FTIR microscope. Microscope apertures ranging from 15 x 15 to as large as 50 x 50 microns were used to define sample areas for analysis.

Analysis was performed using a FT/IR-620 with IRT-30 FTIR microscope with a ceramic source, KBr beamsplitter, and MCT detector. The resolution was 4 cm-1, with 64 averaged scans for the single-beam background and sample spectra. The spectral range was from 4000-700 cm-1.
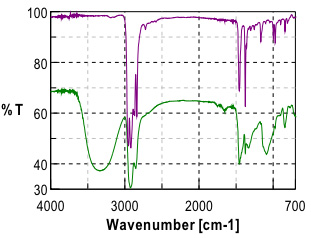
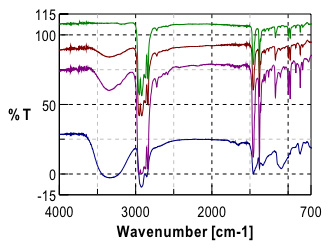
Initial investigation of the polymer laminate found that the primary components of the laminate were Poly(propylene) (PP) and poly(vinyl alcohol) (PVOH). Further study and careful examination of the laminate polymer immediately adjacent to the PVOH layer revealed a gradient in the ratio of the poly(propylene) (PP) and poly(vinyl alcohol)(PVOH) polymers.
Conclusion
The laminate appears to comprise 6 layers. PP (polypropylene) and a highly hydrolyzed form of the PVOH (polyvinyl alcohol) were identified as the primary components based on the comparison of the infrared spectra to spectral libraries. There appear to be two slightly different PP types; PP1 and PP2 are probably molecular weight variants or PP1 may simply contain a small amount of an additive to provide a change in optical density.
Additionally, it was determined that there was a mixing of the polymers at the interfaces. FTIR microscopy was not only able to identify the layers but also to give information on the polymer mixing occurring at the interface. This valuable information will allow a more accurate determination of the physical properties of the polymer and how it will handle stresses at the interface.
Featured Products:

FTIR Microscopy for the Analysis of Polymer Laminates in Beverage Packaging
Introduction
Laminates are combinations of polymers that combine the benefits and properties of the various polymers used in the laminate to create a better material. Polymer laminates have a multitude of uses including packaging for food and other products. Laminates act as moisture and/or oxygen barriers and as such are commonly used to protect foods and chemicals. Analysis of laminates is often required for quality control and to determine laminate adhesion problems.
Modern polymers are seldom used as pure materials, more often mixtures of different polymers are combined to give the desired physical characteristics. For example, the rigidity of polystyrene is often combined with an elastic polymer to produce stiff yet shock resistant materials. Polymeric laminates are important in the microelectronics, medical, food packaging, and chemical industries. Since, laminates act as moisture and/or oxygen barriers they are commonly used to protect foods and chemicals.
There are many methods that can be used to generate these mixtures but one of the most common is to simply assemble the polymers into layers and create a laminate. The most widely used structural spectroscopic measurement for laminates is Fourier Transform infrared (FTIR). FTIR spectroscopy is a non-destructive technique that that allows chemical identification of the component materials in the laminate.
Infrared spectroscopy provides the ability to study the interactions of the vibrational and rotational energies of atoms or groups of atoms within molecules. Infrared spectra reflect vibrational motions that produce a change in the permanent dipole moment of the molecule. Infrared spectroscopy is a powerful qualitative and quantitative tool. There is a large amount of information that can be gained by using infrared spectroscopy for the analysis of polymers. Minor changes to the molecular structure usually result in a spectrum that is clearly distinguishable from the spectrum of the original compound. Thus, they can be used to identify the presence of a specific chemical compound or mixture of compounds.
Experimental
Several different polymer materials were selected for analysis including a prepared juice bottle and a plastic food container. Laminate samples of controlled thickness were sectioned with a razor blade and the cross section examined using the FTIR microscope. Microscope apertures ranging from 15 x 15 to as large as 50 x 50 microns were used to define sample areas for analysis.

Analysis was performed using a FT/IR-620 with IRT-30 FTIR microscope with a ceramic source, KBr beamsplitter, and MCT detector. The resolution was 4 cm-1, with 64 averaged scans for the single-beam background and sample spectra. The spectral range was from 4000-700 cm-1.


Initial investigation of the polymer laminate found that the primary components of the laminate were Poly(propylene) (PP) and poly(vinyl alcohol) (PVOH). Further study and careful examination of the laminate polymer immediately adjacent to the PVOH layer revealed a gradient in the ratio of the poly(propylene) (PP) and poly(vinyl alcohol)(PVOH) polymers.
Conclusion
The laminate appears to comprise 6 layers. PP (polypropylene) and a highly hydrolyzed form of the PVOH (polyvinyl alcohol) were identified as the primary components based on the comparison of the infrared spectra to spectral libraries. There appear to be two slightly different PP types; PP1 and PP2 are probably molecular weight variants or PP1 may simply contain a small amount of an additive to provide a change in optical density.
Additionally, it was determined that there was a mixing of the polymers at the interfaces. FTIR microscopy was not only able to identify the layers but also to give information on the polymer mixing occurring at the interface. This valuable information will allow a more accurate determination of the physical properties of the polymer and how it will handle stresses at the interface.

 Download This Application
Download This Application
