Evaluation of Aggregation-induced Circularly Polarized Luminescence (AICPL) Properties of Platinum Complexes using a CPL Measurement System and Spectrofluorometer
October 16, 2025Introduction
Abstract
Compounds with circularly polarized luminescence (CPL) have attracted attention because of their potential usage as light-emitting diodes in 3D display technology and sensing probes in biological imaging. Many cases rely on the compound being aggregated or in solid form and it is important to demonstrate that the compound still exhibits CPL when aggregated (AICPL). AICPL characterization is performed on samples in the solid state, such as a powders or films, or in an aggregated state, using a CPL measurement system with 180-degree optical configuration. Simultaneously aggregate induced emission (AIE) can be characterized with a standard spectrofluorometer by purposely aggregating samples in solution. Using the evaluation method described here, we report the AICPL properties of platinum complexes in the solid-state using a CPL-300 with 180-degree configuration and the AIE properties of the same complex using a FP-8550 spectrofluorometer.
Introduction
Compounds with circularly polarized luminescence (CPL) have attracted attention because of their potential usage as light-emitting diodes in 3D display technology and sensing probes in biological imaging. In these cases, the practical application of materials requires that the compounds exhibit CPL and sufficient luminescence intensity even in an aggregated state. However, conventional fluorescent molecules have been reported to exhibit aggregation-caused quenching (ACQ), in which the luminescence intensity decreases significantly or disappears completely due to the formation of aggregates in the solid state or in poor solvents. In 2001, B.Z. Tang et al. reported the phenomenon of aggregation-induced emission (AIE), in which the luminescence is not observed in the solution state, but in the aggregated state.1 Since then, research on compounds with AIE properties has been actively performed as a countermeasure against ACQ. Consequently, in recent years, research on compounds with aggregation-induced circularly polarized luminescence (AICPL) properties, in which CPL is enhanced in the aggregate state, has been gaining traction.
To characterize AIE of newly synthesized compounds, solutions are prepared with increasing volume ratios of poor solvent to good solvent in a stepwise manner. The luminescence of the solutions are then measured using a spectrofluorometer to confirm that the luminescence intensity increases with the formation of aggregates when the poor solvent exceeds a certain percentage. AICPL characterization is performed on samples in the solid state, such as a powders or films, or in an aggregated state, using a CPL measurement system. When performing CPL measurements on solid samples, it is necessary to use a 180-degree optical configuration instead of a 90-degree configuration, which is generally used in spectrofluorometers, to take into account the possibility of fluorescence anisotropy of the sample (Figure 1). Using the evaluation method described above, we report the AICPL properties of platinum complexes in the solid-state using a CPL-300 with 180-degree configuration and the AIE properties of the same complex using a FP-8550 spectrofluorometer.
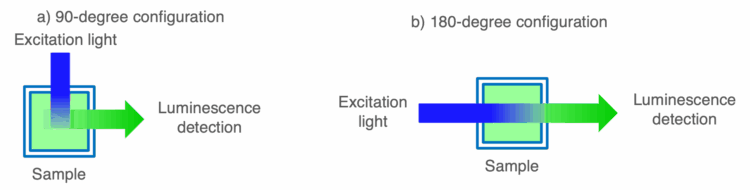
Experimental
Sample
Complexes R-1 and S-1 were based on previously reported chiral platinum complexes with AICPL properties,2) but additional phenol groups were added (Figure 2).
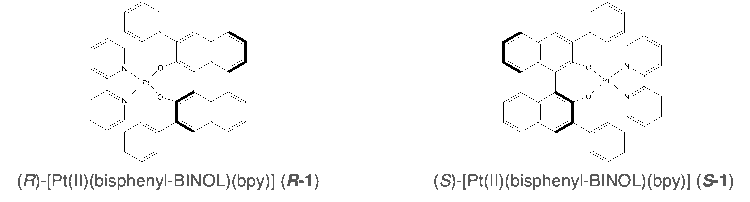
Procedure
- AIE Evaluation
The R-1 concentration was kept at 1 × 10-4 mol/L for each sample. Samples consisted of different solvent mixtures where THF, a good solvent, was mixed with a specific fraction of water (fw): 0, 30, 50, 60, 70, 80, and 90%. 500 μL of each sample was injected into a 5 mm square cell and the luminescence spectra were measured using FP-8550.
- AICPL Evaluation
Powder samples were prepared by dropping an ethanol suspension of R-1 or S-1 onto a quartz plate and allowing the ethanol to evaporate naturally. CPL spectra of the powder samples were then measured using CPL-300.
Parameters
- AIE Evaluation
Ex. wavelength: 350 nm Scanning Speed: 100 nm/min Ex. Bandwidth 5 nm Em. Bandwidth 5 nm Response: 0.5 sec Data Interval: 0.1 nm Sensitivity: Medium -
AICPL Evaluation
Ex. Wavelength 350 nm Scanning Speed: 100 nm/min Ex. Bandwidth 100 nm Em. Bandwidth 100 nm Response: 4 sec Data Interval 0.1 nm Accumulation Time: 9 HT Voltage: 850 v
System
- AIE Evaluation
Instrument: FP-8550 Spectrofluorometer

Accessories: FMH-802 5 mm Micro cell jacket, Micro rectangular FL quartz cell 5×5 mm
- AICPL Evaluation
Instrument: CPL-300 CPL measurement system

Accessories: Solid sample holder
Keywords
AICPL, Aggregation-induced circularly polarized luminescence, AIE, Aggregation-induced emission, CPL, Circularly polarized luminescence, Spectrofluorometer
Results
- AIE Evaluation
The luminescence spectra of R-1 for different fw and the relationship between fw and luminescence intensity at 625 nm are shown in Figure. 3a and Figure. 3b, respectively. No luminescence bands are observed in conditions of fw ≤ 50%, but clear luminescence bands are observed when fw ≥ 60% as the sample aggregates. This result demonstrates that the platinum complexes used in this experiment have AIE properties.
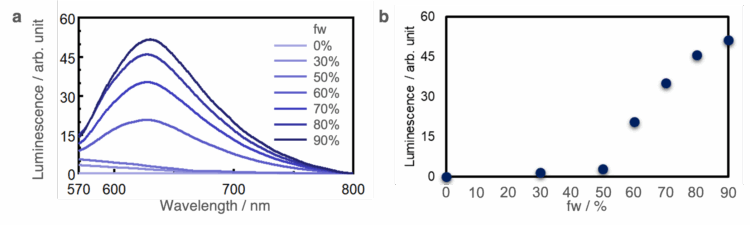
-
AICPL Evaluation
The CPL spectra of R-1 and S-1 powders (Figure 4a) are mirror-images indicating that these complexes exhibit AICPL characteristics. In addition, there are concerns about the effects of artifacts due to linear dichroism and birefringence in CPL measurements of solid samples. Since the spectra have a mirror-image relationship, it can be concluded that the observed CPL signal originates from the sample.
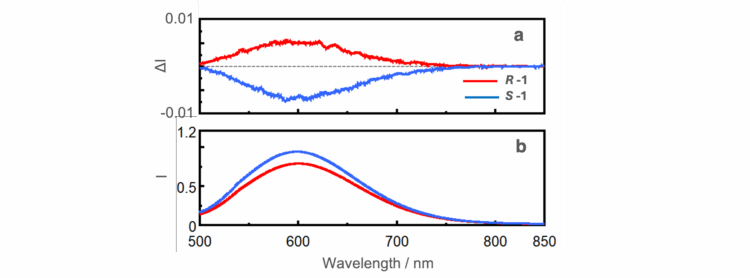
Conclusion
The chiral platinum complexes used in this experiment exhibit AIE and AICPL properties as shown using FP-8550 and CPL-300 spectrometers. The utilization of CPL-300 with 180-degree optical configuration enables the evaluation of AICPL characteristics in the solid state, which is otherwise not possible.
References
This work was performed under the guidance of Prof. Naoto Ishikawa, Graduate School of Science, Osaka University and Prof. Hiroyuki Nishikawa, Faculty of Science, Ibaraki University.
- J. Luo, Z. Xie, J.W.Y. Lam, L. Cheng, H. Chen, C. Qiu, H.S. Kwok, X. Zhan, Y. Liu, D. Zhu, B.Z. Tang: Chemical Communications, 381, 1740–1741 (2001) DOI: 10.1039/B105159H
- D. Tauchi, T. Koida, Y. Nojima, M. Hasegawa, Y. Mazaki, A. Inagaki, K. Sugiura, Y. Nagaya, K. Tsubaki, T. Shiga, Y. Nagata, H. Nishikawa: Chemical Communications, 59, 4004–4007 (2023) DOI: 10.1039/D2CC06198H

 Download This Application
Download This Application