Analyzing the Interaction of Human Serum Albumin and 3,5-Diiodosalicylic Acid
January 5, 2024
Introduction
Human serum albumin (HSA) is the most abundant protein in blood plasma. HSA binds with pharmaceutical compounds and other in-vivo substances and plays an important role in the transport of these substances to target organs. Several studies have reported on the binding affinity of HSA and its interactions with a variety of compounds.
This application notes demonstrates the use of the J-1500 CD spectrometer and ATS-530 automatic titrator to monitor the interaction of HSA titrated with 3,5-diiodosalicylic acid. While 3,5-diiodosalicylic acid is achiral, its interaction with chiral HSA induces circular dichroism.

Experimental
| Measurement Conditions | |
|---|---|
| Data Acquisition Interval | 2 sec |
| Scan Speed | 100 nm/min |
| Spectral Bandwidth | 1 nm |
| Data Pitch | 0.1 nm |
| Path Length | 10 mm |
| Accumulations | 2 |
50 mL aliquots of 0.025 mM of 3,5-diiodosalicylic acid was added to 2 mL of 0.0228 mM of HSA in 100 mM acetate buffer (pH 6.3) 20 times. The CD spectrum was scanned from 360 to 260 nm and also monitored at 320 nm.
Keywords
200-CD-0014, J-1500, Circular Dichroism, CD, HSA, pharmaceuticals, ATS-530 automatic titrator, biochemistry, pharmaceutical
Results
Chirality can be induced in an achiral substance interacting with a chiral substance. This interaction will exhibit circular dichroism. The resulting CD spectrum from the interaction of achiral 3,5-diiodosalicylic acid with chiral HSA shows a positive peak at 320 nm in Figure 1. While HSA does not show a CD signal at 320 nm, the induced CD from the interaction of HSA and 3,5-diiodosalicylic acid depicts this signal at 320 nm which increases with the increasing additions of 3,5-diiodosalicylic acid and is shown in Figure 2.
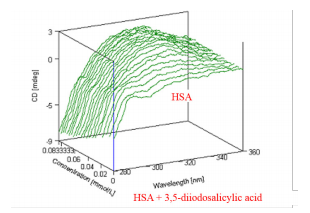
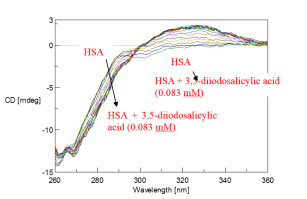
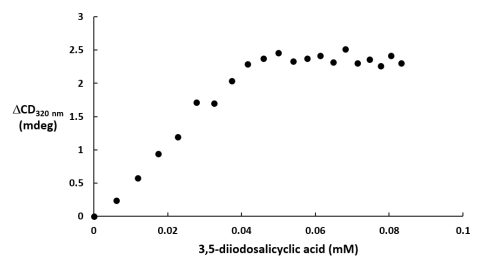
The increase in the induced CD signal at 320 nm was plotted in Figure 3 as a funtion of the 3,5-diiodosalicylic acid concentration. Figure 3 is also known as a Hill plot which describes the cooperativity of the interaction between HSA and 3,5-diiodosalicylic acid. A dissociation constant, which is the concentration at which 50% of a subtsance is bound, can also be estimated from a Hill plot. Using Figure 3, the dissociation constant of 3,5-diiodosalicylic acid binding to HSA was determined to be 0.023 mM. The Hill coefficient is approximately 3.1 which indicates a positive cooperative reaction.
Conclusion
This application note demonstrates the use of the ATS-530 automatic titrator and J-1500 CD spectrometer to obtain CD spectra of the titration of 3,5-diiodosalicylic to HSA in order to determine a dissociation constant.
References
1. R. Brodersen, J. Biol. Chem. (1977) 252, 14, 5067-5072.
2. U. Kragh-Hansen, Biochem. J. (1983) 209, 135-142.
3. Sinha, S. S., Mitra, R. K., and S. K. Pal, J. Phys. Chem.B. (2008) 112, 4884-4891
Featured Products:
-
High-Throughput CD
-

Highest performance with a wide range of accessories for maximum flexibility to meet complex research demands.
J-1500
-
J-1700

Analyzing the Interaction of Human Serum Albumin and 3,5-Diiodosalicylic Acid
Introduction
Human serum albumin (HSA) is the most abundant protein in blood plasma. HSA binds with pharmaceutical compounds and other in-vivo substances and plays an important role in the transport of these substances to target organs. Several studies have reported on the binding affinity of HSA and its interactions with a variety of compounds.
This application notes demonstrates the use of the J-1500 CD spectrometer and ATS-530 automatic titrator to monitor the interaction of HSA titrated with 3,5-diiodosalicylic acid. While 3,5-diiodosalicylic acid is achiral, its interaction with chiral HSA induces circular dichroism.

Experimental
| Measurement Conditions | |
|---|---|
| Data Acquisition Interval | 2 sec |
| Scan Speed | 100 nm/min |
| Spectral Bandwidth | 1 nm |
| Data Pitch | 0.1 nm |
| Path Length | 10 mm |
| Accumulations | 2 |
50 mL aliquots of 0.025 mM of 3,5-diiodosalicylic acid was added to 2 mL of 0.0228 mM of HSA in 100 mM acetate buffer (pH 6.3) 20 times. The CD spectrum was scanned from 360 to 260 nm and also monitored at 320 nm.
Keywords
200-CD-0014, J-1500, Circular Dichroism, CD, HSA, pharmaceuticals, ATS-530 automatic titrator, biochemistry, pharmaceutical
Results
Chirality can be induced in an achiral substance interacting with a chiral substance. This interaction will exhibit circular dichroism. The resulting CD spectrum from the interaction of achiral 3,5-diiodosalicylic acid with chiral HSA shows a positive peak at 320 nm in Figure 1. While HSA does not show a CD signal at 320 nm, the induced CD from the interaction of HSA and 3,5-diiodosalicylic acid depicts this signal at 320 nm which increases with the increasing additions of 3,5-diiodosalicylic acid and is shown in Figure 2.


The increase in the induced CD signal at 320 nm was plotted in Figure 3 as a funtion of the 3,5-diiodosalicylic acid concentration. Figure 3 is also known as a Hill plot which describes the cooperativity of the interaction between HSA and 3,5-diiodosalicylic acid. A dissociation constant, which is the concentration at which 50% of a subtsance is bound, can also be estimated from a Hill plot. Using Figure 3, the dissociation constant of 3,5-diiodosalicylic acid binding to HSA was determined to be 0.023 mM. The Hill coefficient is approximately 3.1 which indicates a positive cooperative reaction.

Conclusion
This application note demonstrates the use of the ATS-530 automatic titrator and J-1500 CD spectrometer to obtain CD spectra of the titration of 3,5-diiodosalicylic to HSA in order to determine a dissociation constant.
References
1. R. Brodersen, J. Biol. Chem. (1977) 252, 14, 5067-5072.
2. U. Kragh-Hansen, Biochem. J. (1983) 209, 135-142.
3. Sinha, S. S., Mitra, R. K., and S. K. Pal, J. Phys. Chem.B. (2008) 112, 4884-4891

 Download This Application
Download This Application
