Highly Sensitive and Comprehensive Detection for Chiral Separation of Pesticides using HPLC-CD-MS
January 5, 2024
Introduction
In this study, we developed a method for chiral separation of pesticides using HPLC-CD-MS, with circular dichroism detector (CD) and mass spectrometer (MS). About 30% of pesticides currently used worldwide are chiral isomers with one or more chiral centers. It is well known that the enantiomers of chiral pesticides have different biological activities, toxicity on non-targeted organisms, rate of metabolism, and biodegradation in the environment. Although it is desirable to use the more effective and safer enantiomers, most chiral pesticides are marketed and used as racemates due to the complicated process and high cost of manufacturing a single enantiomer molecule.
In general, chiral separation by HPLC, GC, and SFC has been one of the most important ways for analyzing the synthetic approach to individual enantiomers. However, HPLC still dominates chromatographic chiral analysis because of its ease of use.
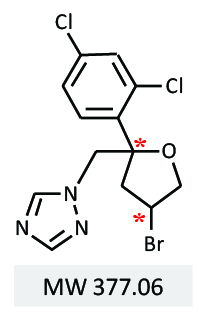
A CD detector simultaneously provides circular dichroism and UV absorbance data, and enables selective identification of the CD polarity of each enantiomer. MS spectra are used to distinguish between chiral isomer groups with the same m/z from other components including impurities. Highly sensitive and comprehensive detection with UV, CD, and MS contributes to the widely applicable and highly accurate analysis for chiral separation in the efficient development of a single enantiomer pesticide. The system and chiral separation method described here is applicable to many industries that deal with chiral compounds. In this presentation, we will present the application of HPLC to bromuconazole, a type of triazole fungicide (Figure 1).
Experimental
Apparatus
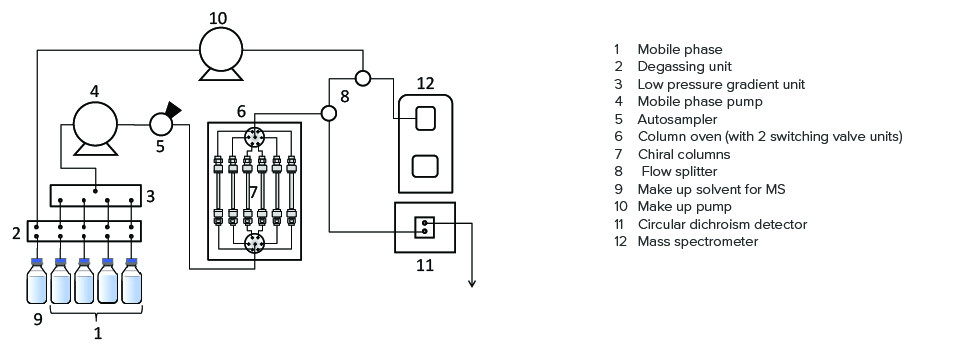
Figure 2 details the JASCO HPLC-CD-MS system used in this experiment, together with Figure 3 that shows the schematic diagram of the system. Using a splitter with a PEEK-coated capillary tube, the flow from a chiral column is divided into two channels, one each to the CD and MS detectors. The CD detector provides CD and UV signals simultaneously. This system enables automated method scouting analysis to be performed on mobile phases and columns.

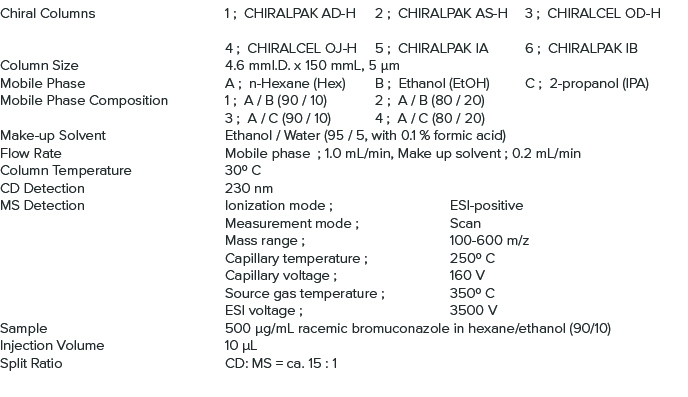
Analysis Conditions for Method Scouting
Keywords
101021H
Results
Results of Method Scouting on Mobile Phases and Columns
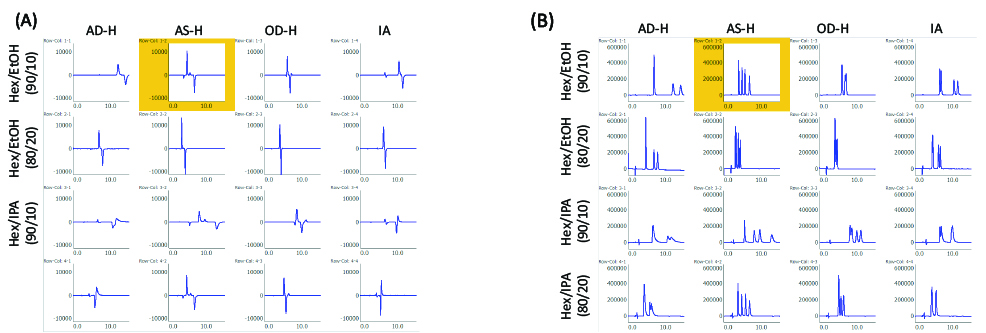
Racemic bromuconazole with 2 chiral centers can be separated into 4 isomer peaks using a chiral column. Figure 4 shows the selected results of method scouting analysis for mobile phases and columns. The best separation was observed with the combination of n-hexane/ethanol (90/10) and CHIRALPAK AS-H (highlighted in yellow). This separation condition was used in the following experiments.
Comprehensive Detection by CD and MS Detectors
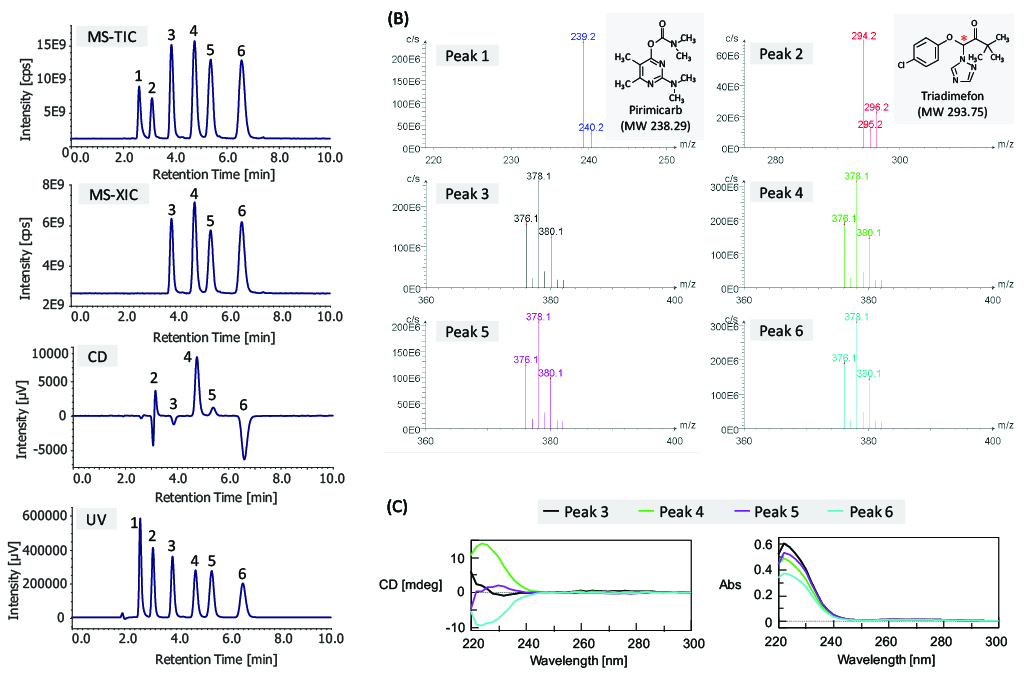
Figure 5 shows the results of bromuconazole standard spiked with two additional pesticides, pirimicarb and triadimefon, as examples of contaminants detected by CD and MS detectors. Figure 5A shows the total ion chromatogram (TIC) from 100 to 600 m/z, extracted ion chromatogram (XIC) of m/z 378.1, CD and UV chromatograms. Figure 5B shows MS spectra of each peak described in Figure 5A. As shown in this figure, we can clearly discriminate the bromuconazole isomers and other contaminants. Bromuconazole isomers (peaks 3 ~ 6) have similarities in their spectra, and a protonated molecular ion ([M+H]+) was observed at m/z 378.1 as a base peak in the mass spectrum of each isomer peak. Protonated molecular ions of pirimicarb and triadimefon are also observed at m/z 239.2 (peak 1) and m/z 294.2 (peak 2). Figure 5C shows the stopped-flow scanned spectra of CD and UV for bromuconazole standard peaks (peaks 3 ~ 6). According to the CD information in Figure 5A and Figure 5C, peaks 3 and 5 of the bromuconazole isomers are estimated to be an enantiomeric pair from their CD polarity and wavelength maxima. Peaks 4 and 6 are also the same. Furthermore, peak 2 in the CD chromatogram in Figure 5A indicates the possibility that can be each enantiomer of triadimefon can be separated.
In this way, comprehensive detection by CD and MS detectors is useful to identify the target chiral isomers group and other contaminants with chiral separation.
Linearity, Reproducibility and Sensitivity
Figure 6 shows the overlaid chromatograms of SIM (Single Ion Monitoring) at m/z 378.1, CD, and UV of bromuconazole standards (2.5, 12.5, and 25 µg/mL enantiomer concentration). Table 1 shows the linearity, reproducibility of retention time and peak area, and detection limit of the bromuconazole enantiomers (the concentrations are for enantiomers). As shown by these results, a wide linear dynamic range and highly sensitive detection were obtained, especially for the SIM chromatogram.
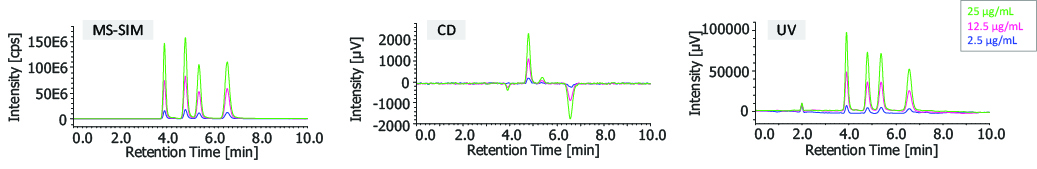
Table 1. Linearity, reproducibility of retention time and peak area, and detection limit of bromuconazole enantiomers
| %RSD (n=10) | ||||
| Correlation Coefficent | tR | Peak Area | Detection Limit (S/N=3) |
|
| MS-SIM (m/z 378.1) | 0.9994 ~ 0.9997 (0.025 ~ 25 µg/mL) | 0.06 ~ 0.11*1 | 1.51 ~ 1.99*1 | 0.0034 ~ 0.0057 µg/mL*3 |
| CD | 0.9987 ~ 0.9994 (12.5 ~ 125 µg/mL) | 0.06 ~ 0.14*2 | 0.86 ~ 2.64*2 | 0.55 ~ 5.06 µg/mL*4 |
| UV | 0.9992 ~ 09997 (2.5 ~ 125 µg/mL) | 0.06 ~ 0.11*2 | 1.11 ~ 1.31*2 | 0.089 ~ 0.16 µg/mL*4 |
*1 Calculated from the results of 1.25 µg/mL.
*2 Calculated from the results of 62.5 µg/mL.
*3 Calculated from the results of 0.125 µg/mL.
*4 Calculated from the results of 25 µg/mL.
Conclusion
We developed a chiral separation method for pesticides by HPLC with CD and MS detection.
Detection by TIC, CD, and UV enable identification of the target chiral isomers group and other contaminants with chiral separation.
Wide dynamic range and high sensitivity for each bromuconazole enantiomer were obtained, especially in the SIM chromatogram.
This system is applicable to many industries that deal with chiral compounds.
References
B. S. Sekhon, J. Pestic. Sci, 2009, 34 (1), 1-12.
V. Pérez-Fernández, M. Á. Garciá, M. L. Marina, J. Chromatogr. A, 2011, 1218, 6561-6582.
Featured Products:

Highly Sensitive and Comprehensive Detection for Chiral Separation of Pesticides using HPLC-CD-MS
Introduction
In this study, we developed a method for chiral separation of pesticides using HPLC-CD-MS, with circular dichroism detector (CD) and mass spectrometer (MS). About 30% of pesticides currently used worldwide are chiral isomers with one or more chiral centers. It is well known that the enantiomers of chiral pesticides have different biological activities, toxicity on non-targeted organisms, rate of metabolism, and biodegradation in the environment. Although it is desirable to use the more effective and safer enantiomers, most chiral pesticides are marketed and used as racemates due to the complicated process and high cost of manufacturing a single enantiomer molecule.
In general, chiral separation by HPLC, GC, and SFC has been one of the most important ways for analyzing the synthetic approach to individual enantiomers. However, HPLC still dominates chromatographic chiral analysis because of its ease of use.
A CD detector simultaneously provides circular dichroism and UV absorbance data, and enables selective identification of the CD polarity of each enantiomer. MS spectra are used to distinguish between chiral isomer groups with the same m/z from other components including impurities. Highly sensitive and comprehensive detection with UV, CD, and MS contributes to the widely applicable and highly accurate analysis for chiral separation in the efficient development of a single enantiomer pesticide. The system and chiral separation method described here is applicable to many industries that deal with chiral compounds. In this presentation, we will present the application of HPLC to bromuconazole, a type of triazole fungicide (Figure 1).

Experimental
Apparatus
Figure 2 details the JASCO HPLC-CD-MS system used in this experiment, together with Figure 3 that shows the schematic diagram of the system. Using a splitter with a PEEK-coated capillary tube, the flow from a chiral column is divided into two channels, one each to the CD and MS detectors. The CD detector provides CD and UV signals simultaneously. This system enables automated method scouting analysis to be performed on mobile phases and columns.


Analysis Conditions for Method Scouting

Results
Results of Method Scouting on Mobile Phases and Columns
Racemic bromuconazole with 2 chiral centers can be separated into 4 isomer peaks using a chiral column. Figure 4 shows the selected results of method scouting analysis for mobile phases and columns. The best separation was observed with the combination of n-hexane/ethanol (90/10) and CHIRALPAK AS-H (highlighted in yellow). This separation condition was used in the following experiments.

Comprehensive Detection by CD and MS Detectors
Figure 5 shows the results of bromuconazole standard spiked with two additional pesticides, pirimicarb and triadimefon, as examples of contaminants detected by CD and MS detectors. Figure 5A shows the total ion chromatogram (TIC) from 100 to 600 m/z, extracted ion chromatogram (XIC) of m/z 378.1, CD and UV chromatograms. Figure 5B shows MS spectra of each peak described in Figure 5A. As shown in this figure, we can clearly discriminate the bromuconazole isomers and other contaminants. Bromuconazole isomers (peaks 3 ~ 6) have similarities in their spectra, and a protonated molecular ion ([M+H]+) was observed at m/z 378.1 as a base peak in the mass spectrum of each isomer peak. Protonated molecular ions of pirimicarb and triadimefon are also observed at m/z 239.2 (peak 1) and m/z 294.2 (peak 2). Figure 5C shows the stopped-flow scanned spectra of CD and UV for bromuconazole standard peaks (peaks 3 ~ 6). According to the CD information in Figure 5A and Figure 5C, peaks 3 and 5 of the bromuconazole isomers are estimated to be an enantiomeric pair from their CD polarity and wavelength maxima. Peaks 4 and 6 are also the same. Furthermore, peak 2 in the CD chromatogram in Figure 5A indicates the possibility that can be each enantiomer of triadimefon can be separated.
In this way, comprehensive detection by CD and MS detectors is useful to identify the target chiral isomers group and other contaminants with chiral separation.

Linearity, Reproducibility and Sensitivity
Figure 6 shows the overlaid chromatograms of SIM (Single Ion Monitoring) at m/z 378.1, CD, and UV of bromuconazole standards (2.5, 12.5, and 25 µg/mL enantiomer concentration). Table 1 shows the linearity, reproducibility of retention time and peak area, and detection limit of the bromuconazole enantiomers (the concentrations are for enantiomers). As shown by these results, a wide linear dynamic range and highly sensitive detection were obtained, especially for the SIM chromatogram.

Table 1. Linearity, reproducibility of retention time and peak area, and detection limit of bromuconazole enantiomers
| %RSD (n=10) | ||||
| Correlation Coefficent | tR | Peak Area | Detection Limit (S/N=3) |
|
| MS-SIM (m/z 378.1) | 0.9994 ~ 0.9997 (0.025 ~ 25 µg/mL) | 0.06 ~ 0.11*1 | 1.51 ~ 1.99*1 | 0.0034 ~ 0.0057 µg/mL*3 |
| CD | 0.9987 ~ 0.9994 (12.5 ~ 125 µg/mL) | 0.06 ~ 0.14*2 | 0.86 ~ 2.64*2 | 0.55 ~ 5.06 µg/mL*4 |
| UV | 0.9992 ~ 09997 (2.5 ~ 125 µg/mL) | 0.06 ~ 0.11*2 | 1.11 ~ 1.31*2 | 0.089 ~ 0.16 µg/mL*4 |
*1 Calculated from the results of 1.25 µg/mL.
*2 Calculated from the results of 62.5 µg/mL.
*3 Calculated from the results of 0.125 µg/mL.
*4 Calculated from the results of 25 µg/mL.
Conclusion
We developed a chiral separation method for pesticides by HPLC with CD and MS detection.
Detection by TIC, CD, and UV enable identification of the target chiral isomers group and other contaminants with chiral separation.
Wide dynamic range and high sensitivity for each bromuconazole enantiomer were obtained, especially in the SIM chromatogram.
This system is applicable to many industries that deal with chiral compounds.
Keywords
101021H
References
B. S. Sekhon, J. Pestic. Sci, 2009, 34 (1), 1-12.
V. Pérez-Fernández, M. Á. Garciá, M. L. Marina, J. Chromatogr. A, 2011, 1218, 6561-6582.

 Download This Application
Download This Application

