Chromatographic Analysis of Allethrin Isomers: A Synthetic Pyrethroid Pesticide
October 8, 2024Introduction
The widespread use of pesticides can adversely affect human health and the environment. Allethrin is a synthetic pesticide belonging to the pyrethroid family. Allethrin causes paralysis and death of insects by targeting their nervous system. Mosquitoes, flies, cockroaches, and ants are all effectively controlled by it. Several formulations of allethrin are available for domestic use, such as sprays, coils, and heated mats. Allethrin’s chemical structure is like that of pyrethrins found in chrysanthemums. However, allethrin is much more stable and has a longer residual effect than natural pyrethrins. The long-term stability of allethrin makes it a more effective insecticide but relatively more persistent in the environment.
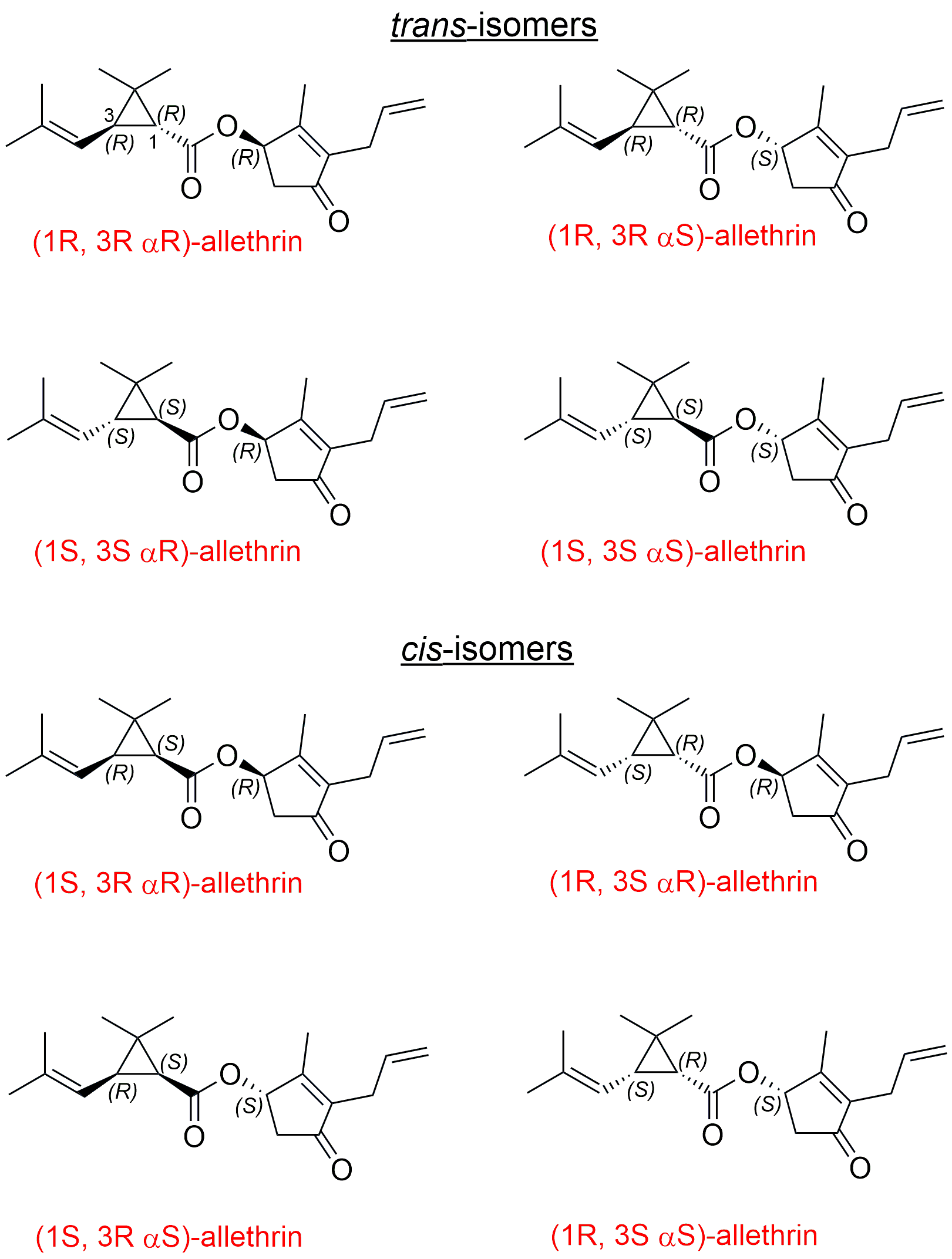
Given the presence of three chiral centers in allethrin (Figure 1), eight stereoisomers are expected. Figure 1 shows all the possible stereoisomers, and commercial allethrin standards contain all eight isomers. However, separating all pyrethroid isomers in a single analysis without chemical derivatization or using chiral additives in mobile phases is highly challenging in liquid chromatography because of geometrical and optical isomerism. Usually, it requires a 2D-HPLC system or two different columns in tandem. A 2D normal phase system using JASCO’s instrumentation is described elsewhere.1 This application note addresses these issues by separating as many allethrin isomers as possible in 1D-HPLC. The analysis can be carried out at several levels of isomeric “details” using JASCO’s rapid HPLC (RHPLC) with a photodiode array detector (Figure 2) and a JASCO circular dichroism detector (CD) (Figure 3). In the first step, two reversed phase methods are developed that distinguish (i) the cis- and trans- isomers, (ii) diastereoisomers of the cis- and trans- isomers, (iii) followed by enantiomeric separation of allethrin isomers on a chiral column as verified with (iv) circular dichroism detection.

Experimental

The JASCO RHPLC is equipped with a degasser, binary pump and an active mixer (PU-4180), autosampler (AS-4150) with a 5 µL sample loop, column oven (CO-4062), and photodiode array detector, PDA, (MD 4010). In addition, for circular dichroism detection of eluting enantiomers, JASCO’s CD-4095 detector was employed. Columns: XSelect HSS C18 SB Column (Waters), 100 mm x 4.6 mm i.d. packed with 3.5 µm fully porous particles (FPP), Hypercarb from Thermo Scientific, 100 mm x 3.0 mm i.d. column packed with 3 µm FPP graphitic carbon, CDShell column (AZYP, LLC), 150×4.6 mm i.d. packed with 2.7 µm superficially porous particles (SPP). Chemicals: HPLC grade acetonitrile, deionized water (18.2 MΩ), trifluoroacetic acid (TFA), (Millipore Sigma, USA) and allethrin standard (C19H26O3, CAS Number: 584-79-2) were obtained from Millipore Sigma (USA). Mobile phase compositions refer to volume-by-volume (v/v) compositions.
Keywords
Allethrin-Isomers, Pyrethoid, Pesticide, HPLC, RHPLC, CD, Chiral, Insecticide, Allethrin
Results
Group separation of trans- and cis-allethrin isomers on a C18 column
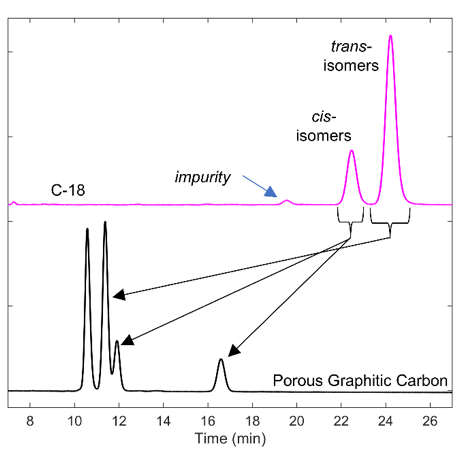
There are different levels of chemical analysis at which one may analyze allethrin samples and standards, as indicated in the introduction. When the sole purpose is quantifying the relative ratio of the cis– and trans– isomers, a high-efficiency C18 isocratic separation is sufficient with JASCO’s PDA detection. Note that not all tested C18 columns could baseline resolve the isomers with ACN and water. Running the X-Select C18 column prior to enantiomeric separation is always beneficial in the case of allethrin. If the sample is impure, some components may co-elute with the analyte of interest, making it difficult to interpret the results obtained from the chiral column. Using a reversed-phase column first allows you to qualitatively understand the impurities and their chromatographic and UV-Vis behavior (Figure 4). The PDA detection also lets you assess the analytes’ signal magnitude by examining the full PDA spectrum in UV-Vis at any wavelength interval of choice with excellent wavelength resolution. The PDA also allows the user to select the best wavelengths, including those values where impurities that do not absorb significantly. The C18 separation of allethrin likewise indicates the presence of small contaminants and two fully resolved major peaks using a straightforward isocratic method with 50:50 ACN: Water containing 0.01% TFA. The area ratio in Figure 4 allowed cis- and trans- peak assignment ( area ratio of 23.10 and 76.90%). These values are comparable with the nominal literature ratio of 20/80 in commercial standards from other sources.1
Separation of allethrin diastereoisomers on a porous graphitic carbon column in reversed-phase mode
Porous graphitic carbon is a superhydrophobic phase (100% carbon) with high shape selectivity and pH stability (1-14). Further group separation of cis- and trans- isomers can be achieved on the Hypercarb column, as shown in Figure 4. A small amount of TFA improves peak shapes by reducing the tailing. It is interesting to note the reversal of selectivity between cis- and trans- isomers on Hypercarb despite using a similar mobile phase as the C18 column. The peak area ratios are as follows: 36.0%, 40.4%, 12.5%, and 11.1% (in the order of elution in Figure 4).
Enantioseparation of allethrin isomers on CD-Shell RSP
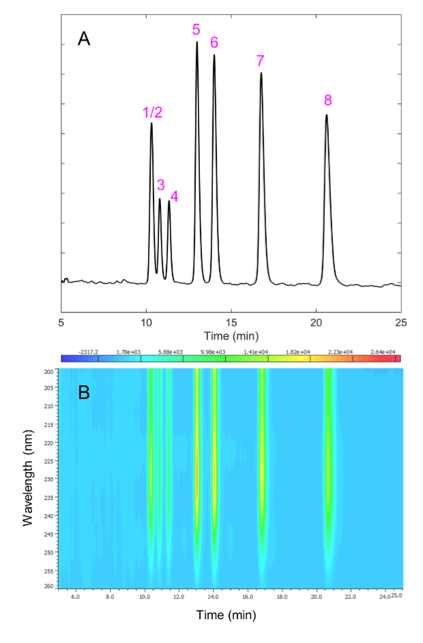
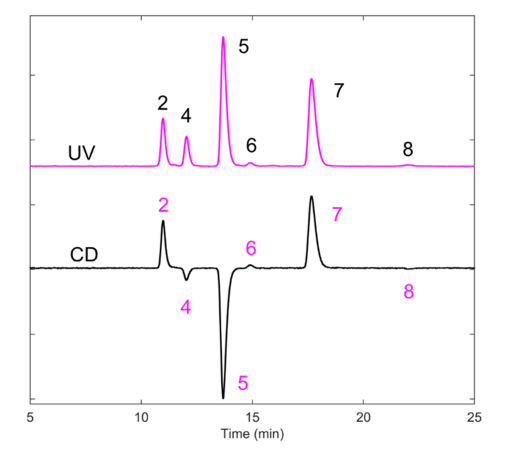
CD-Shell RSP is a high-efficiency chiral column based on 2.7 µm core-shell particles bonded with (2-hydroxypropyl)-β-cyclodextrin. This column chemistry is very shape selective in the reverse-phase mode for allethrin using water and acetonitrile without additives. A CD-Shell RSP column offers rapid enantiomeric separations of allethrin for analysts interested in pursuing enantiomeric separations. The separation of the allethrin isomers (including enantiomers) is shown in Figure 5A. Note that seven of the eight isomers are separated, with the first peak containing two enantiomers. The PDA shows similar spectrum shapes in Figure 5B for each isomer. Interestingly, CD detection in Figure 6 shows all isomers indicating slight chiral discrimination in the first peak.
Calibration curve linearity on JASCO’s RHPLC with PDA detector
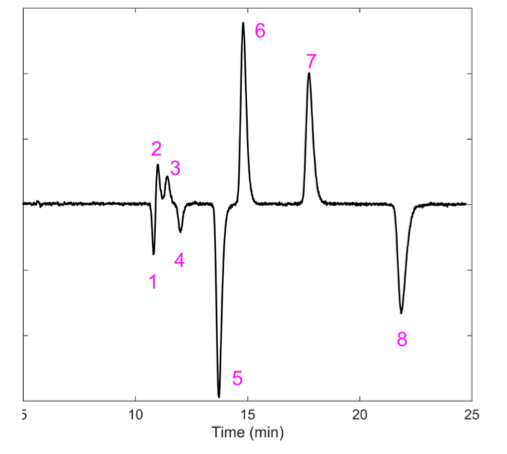
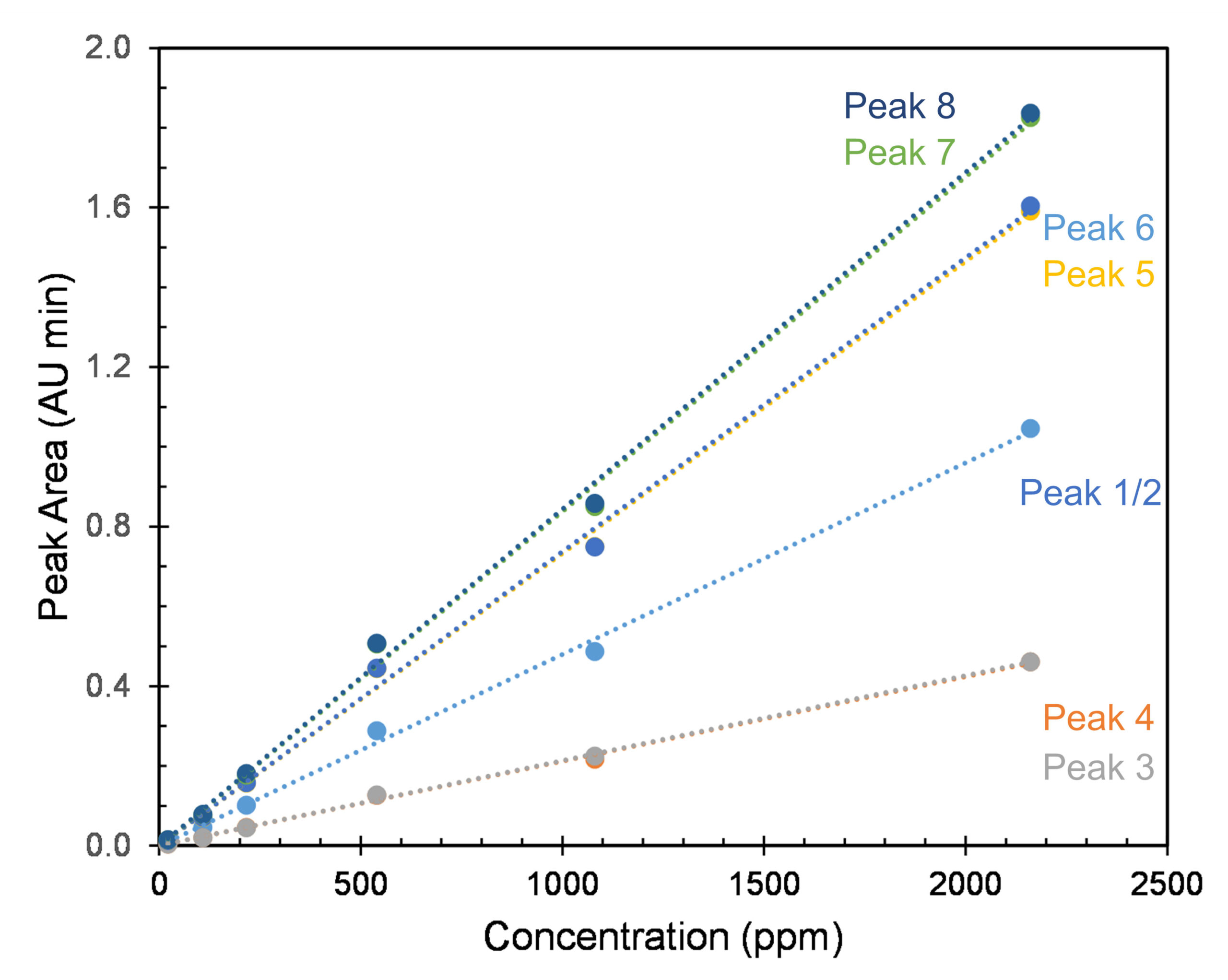
In order to test the linearity of the CD-Shell RSP method, a series of allethrin standards were made, ranging from 21.6 to 2160 mg/L in methanol. The concentration on the x-axis is for the total allethrin concentration. Satisfactory linearity was achieved throughout this range, as shown in Figure 7. Extraction of commercial mosquito repellent mats was done using methanol. The repellant mats contain 21.97% d- cis/trans allethrin with other undisclosed ingredients. Figure 8 shows the enantioseparations with UV and CD detection. Here peaks 1 and 3 are completely missing, and peaks 6 and 8 are present in trace amounts.
Conclusion
Synthetic pyrethroids are among the most challenging chromatographic separations because of their large number of isomers. Therefore, a C18 column, a shape-selective achiral column, and a derivatized cyclodextrin column were employed to analyze the isomeric ratios. JASCO’s PDA detection, followed by circular dichroism detection, allows researchers to conveniently investigate isomeric ratios of allethrin using 1D HPLC systems, all in reversed-phase using ACN/Water mobile phases.
References
1. Mancini, F., Fiori, J., Bertucci, C., Cavrini, V., Bragieri, M., Zanotti, M.C., Liverani, A., Borzatta, V. and Andrisano, V., 2004. Stereoselective determination of allethrin by two-dimensional achiral/chiral liquid chromatography with ultraviolet/circular dichroism detection. Journal of Chromatography A, 1046(1-2), pp.67-73.
(Experimentation/figures): Siddharth Jaya Sajeevan J, Troy T. Handlovic
(Report writing, conceptualization, & supervision): M. Farooq Wahab and Daniel W. Armstrong

Chromatographic Analysis of Allethrin Isomers: A Synthetic Pyrethroid Pesticide
Introduction

The widespread use of pesticides can adversely affect human health and the environment. Allethrin is a synthetic pesticide belonging to the pyrethroid family. Allethrin causes paralysis and death of insects by targeting their nervous system. Mosquitoes, flies, cockroaches, and ants are all effectively controlled by it. Several formulations of allethrin are available for domestic use, such as sprays, coils, and heated mats. Allethrin’s chemical structure is like that of pyrethrins found in chrysanthemums. However, allethrin is much more stable and has a longer residual effect than natural pyrethrins. The long-term stability of allethrin makes it a more effective insecticide but relatively more persistent in the environment.
Given the presence of three chiral centers in allethrin (Figure 1), eight stereoisomers are expected. Figure 1 shows all the possible stereoisomers, and commercial allethrin standards contain all eight isomers. However, separating all pyrethroid isomers in a single analysis without chemical derivatization or using chiral additives in mobile phases is highly challenging in liquid chromatography because of geometrical and optical isomerism. Usually, it requires a 2D-HPLC system or two different columns in tandem. A 2D normal phase system using JASCO’s instrumentation is described elsewhere.1 This application note addresses these issues by separating as many allethrin isomers as possible in 1D-HPLC. The analysis can be carried out at several levels of isomeric “details” using JASCO’s rapid HPLC (RHPLC) with a photodiode array detector (Figure 2) and a JASCO circular dichroism detector (CD) (Figure 3). In the first step, two reversed phase methods are developed that distinguish (i) the cis- and trans- isomers, (ii) diastereoisomers of the cis- and trans- isomers, (iii) followed by enantiomeric separation of allethrin isomers on a chiral column as verified with (iv) circular dichroism detection.

Experimental

The JASCO RHPLC is equipped with a degasser, binary pump and an active mixer (PU-4180), autosampler (AS-4150) with a 5 µL sample loop, column oven (CO-4062), and photodiode array detector, PDA, (MD 4010). In addition, for circular dichroism detection of eluting enantiomers, JASCO’s CD-4095 detector was employed. Columns: XSelect HSS C18 SB Column (Waters), 100 mm x 4.6 mm i.d. packed with 3.5 µm fully porous particles (FPP), Hypercarb from Thermo Scientific, 100 mm x 3.0 mm i.d. column packed with 3 µm FPP graphitic carbon, CDShell column (AZYP, LLC), 150×4.6 mm i.d. packed with 2.7 µm superficially porous particles (SPP). Chemicals: HPLC grade acetonitrile, deionized water (18.2 MΩ), trifluoroacetic acid (TFA), (Millipore Sigma, USA) and allethrin standard (C19H26O3, CAS Number: 584-79-2) were obtained from Millipore Sigma (USA). Mobile phase compositions refer to volume-by-volume (v/v) compositions.
Results
Group separation of trans- and cis-allethrin isomers on a C18 column

There are different levels of chemical analysis at which one may analyze allethrin samples and standards, as indicated in the introduction. When the sole purpose is quantifying the relative ratio of the cis– and trans– isomers, a high-efficiency C18 isocratic separation is sufficient with JASCO’s PDA detection. Note that not all tested C18 columns could baseline resolve the isomers with ACN and water. Running the X-Select C18 column prior to enantiomeric separation is always beneficial in the case of allethrin. If the sample is impure, some components may co-elute with the analyte of interest, making it difficult to interpret the results obtained from the chiral column. Using a reversed-phase column first allows you to qualitatively understand the impurities and their chromatographic and UV-Vis behavior (Figure 4). The PDA detection also lets you assess the analytes’ signal magnitude by examining the full PDA spectrum in UV-Vis at any wavelength interval of choice with excellent wavelength resolution. The PDA also allows the user to select the best wavelengths, including those values where impurities that do not absorb significantly. The C18 separation of allethrin likewise indicates the presence of small contaminants and two fully resolved major peaks using a straightforward isocratic method with 50:50 ACN: Water containing 0.01% TFA. The area ratio in Figure 4 allowed cis- and trans- peak assignment ( area ratio of 23.10 and 76.90%). These values are comparable with the nominal literature ratio of 20/80 in commercial standards from other sources.1
Separation of allethrin diastereoisomers on a porous graphitic carbon column in reversed-phase mode
Porous graphitic carbon is a superhydrophobic phase (100% carbon) with high shape selectivity and pH stability (1-14). Further group separation of cis- and trans- isomers can be achieved on the Hypercarb column, as shown in Figure 4. A small amount of TFA improves peak shapes by reducing the tailing. It is interesting to note the reversal of selectivity between cis- and trans- isomers on Hypercarb despite using a similar mobile phase as the C18 column. The peak area ratios are as follows: 36.0%, 40.4%, 12.5%, and 11.1% (in the order of elution in Figure 4).
Enantioseparation of allethrin isomers on CD-Shell RSP
CD-Shell RSP is a high-efficiency chiral column based on 2.7 µm core-shell particles bonded with (2-hydroxypropyl)-β-cyclodextrin. This column chemistry is very shape selective in the reverse-phase mode for allethrin using water and acetonitrile without additives. A CD-Shell RSP column offers rapid enantiomeric separations of allethrin for analysts interested in pursuing enantiomeric separations. The separation of the allethrin isomers (including enantiomers) is shown in Figure 5A. Note that seven of the eight isomers are separated, with the first peak containing two enantiomers. The PDA shows similar spectrum shapes in Figure 5B for each isomer. Interestingly, CD detection in Figure 6 shows all isomers indicating slight chiral discrimination in the first peak.


Calibration curve linearity on JASCO’s RHPLC with PDA detector
In order to test the linearity of the CD-Shell RSP method, a series of allethrin standards were made, ranging from 21.6 to 2160 mg/L in methanol. The concentration on the x-axis is for the total allethrin concentration. Satisfactory linearity was achieved throughout this range, as shown in Figure 7. Extraction of commercial mosquito repellent mats was done using methanol. The repellant mats contain 21.97% d- cis/trans allethrin with other undisclosed ingredients. Figure 8 shows the enantioseparations with UV and CD detection. Here peaks 1 and 3 are completely missing, and peaks 6 and 8 are present in trace amounts.


Conclusion
Synthetic pyrethroids are among the most challenging chromatographic separations because of their large number of isomers. Therefore, a C18 column, a shape-selective achiral column, and a derivatized cyclodextrin column were employed to analyze the isomeric ratios. JASCO’s PDA detection, followed by circular dichroism detection, allows researchers to conveniently investigate isomeric ratios of allethrin using 1D HPLC systems, all in reversed-phase using ACN/Water mobile phases.
Keywords
Allethrin-Isomers, Pyrethoid, Pesticide, HPLC, RHPLC, CD, Chiral, Insecticide, Allethrin
References
1. Mancini, F., Fiori, J., Bertucci, C., Cavrini, V., Bragieri, M., Zanotti, M.C., Liverani, A., Borzatta, V. and Andrisano, V., 2004. Stereoselective determination of allethrin by two-dimensional achiral/chiral liquid chromatography with ultraviolet/circular dichroism detection. Journal of Chromatography A, 1046(1-2), pp.67-73.
(Experimentation/figures): Siddharth Jaya Sajeevan J, Troy T. Handlovic
(Report writing, conceptualization, & supervision): M. Farooq Wahab and Daniel W. Armstrong

 Download This Application
Download This Application