Chromatographic Analysis of Amygdalin Isomers
October 6, 2023Introduction
Cyanogenic glycosides belong to a broad category of plant toxins [1]. The defining feature of these potentially toxic compounds is their ability to produce free hydrogen cyanide, a process called cyanogenesis. The generation of HCN is believed to function as a defense strategy for plants against fungal infections and predation. Since humans consume plants and their fruits and seeds, we can encounter these toxins inadvertently. These compounds appear in various structural forms across the plant world. Amygdalin is the most common cyanogenic glycoside found in seeds and kernels of the Rosaceae (rose family). Specifically, amygdalin, a primary constituent derived from bitter almonds, peaches, apricots, or cherry pits, has been a significant component of traditional Eastern medicine and has been the subject of research for almost two centuries [2]. When these seeds or nuts are eaten, amygdalin comes in contact with hydrolytic enzymes that first produce prunasin (D-mandelonitrile-β-D-glucoside) and glucose and, in a second step, prunasin can be converted into benzaldehyde and hydrogen cyanide, the mixture of these chemicals producing the pleasant almond-like aroma [3].
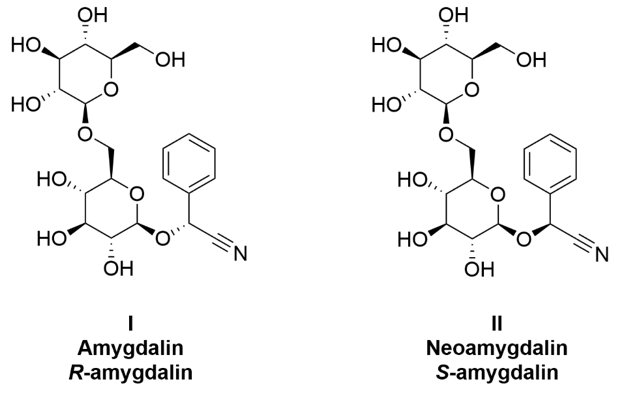
It is important to quantify the amount of HCN precursor, amygdalin, present in food and supplements. Since amygdalin is naturally present in several food items and is currently being marketed online as a nutritional supplement under the questionable designation of Vitamin B17, it is important to be able to monitor it using reliable analytical chromatography. In Figure 1, the structures of amygdalin (I) and its epimer (II) are shown. The compound has eleven stereogenic centers, ten of which are contained in the two glucopyranose moieties and are not easily isomerized. The remaining stereogenic center (i.e., the carbon atom with the phenyl and nitrile substituents) is susceptible to inversion, which can lead to the formation of neoamygdalin. Naturally occurring amygdalin exhibits the (R)-configuration at the phenyl substituted stereogenic center. This chiral center isomerizes in slightly basic conditions or neutral aqueous solutions, yielding the (S)-epimer (II). While the plant-extracted isomer of amygdalin primarily exists as the (R)-epimer, improper storage by companies can rapidly cause interconversion and even decomposition to other related compounds [4]. Amygdalin also shows an interesting behavior: its epimerization rate in aqueous solution depends on the glassware in which the solution is stored [3]. Some scientific inconsistencies are found in published analytical methods that often ignore the problem of amygdalin epimerization in glassware [3]. We establish reliable analytical methods using porous graphitic carbon and a cyclodextrin-based chiral stationary phase in the reversed phase mode to separate the two amygdalin epimers in less than six minutes using JASCO’s rapid HPLC or RHPLC with PDA detection.
Experimental
The JASCO RHPLC is equipped with a degasser, binary pump and an active mixer (PU-4180), autosampler (AS-4150) with a 5 µL sample loop, column oven (CO-4062), and photodiode array detector, PDA, (MD 4010). Columns: Hypercarb from Thermo Scientific, 150 mm x 4.6 mm i.d. column packed with 5 µm fully porous graphitic carbon, CDShell-RSP column (AZYP, LLC, Arlington, Texas), 150×3 mm i.d. packed with 2.7 µm superficially porous particles (SPP). Chemicals: HPLC grade acetonitrile, methanol, deionized water (18 MΩ-cm), and amygdalin standard (CAS No. 29883-15-6) were obtained from Millipore Sigma (USA). The amygdalin is extracted from apricot kernels, and the source code is SLCK7401. Mobile phase compositions refer to volume-by-volume (v/v) compositions. A single pump was used after manually mixing the solvents. The calibration curve solutions were prepared in 1 mL volumetric flasks in deionized water with a trace of acetic acid to prevent epimerization.
Keywords
HPLC, Chromatography, Amygdalin, Neoamygdalin, Cyanogenic Glycosides, Rosaceae, Seeds, Chiral, Epimer, Vitamin B17
Results
Epimerization of Amygdalin
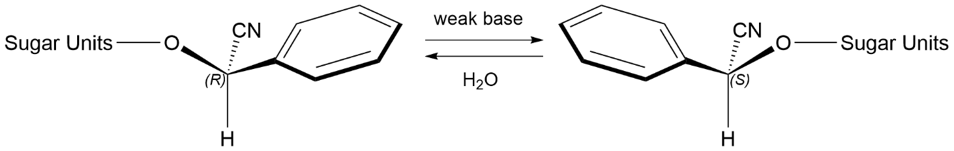
Given the fact that the natural amygdalin in aqueous solutions can rapidly epimerize to neoamygdalin (II), columns must be screened for epimeric selectivity. The epimerization of (I) can be conveniently carried out by adding aqueous ammonia. A typical approach could be measuring 0.05 g of amygdalin in 5 mL of deionized water in a clean glass vial, adding 10 µL of NH3, and allowing about 20 mins to epimerize at room temperature. This process is shown in Figure 2. Under equilibrium conditions, neoamygdalin dominates slightly, typically around 57% to 43%.
Separation of Neoamygdalin and Amygdalin
In the early analytical methods, amygdalin epimers were differentiated through gas chromatography (GC) using trifluoroacetylated derivatives. Subsequently, capillary electrophoresis was employed to distinguish the epimers within 15 minutes, showing commendable selectivity with a cyclodextrin chiral additive in the run buffer. Despite these methods, in most research, liquid chromatography (LC) with a C-18 phase appears to have been the preferred approach, even though it led to overlapping of adjacent peaks for the R– and S– forms of amygdalin.
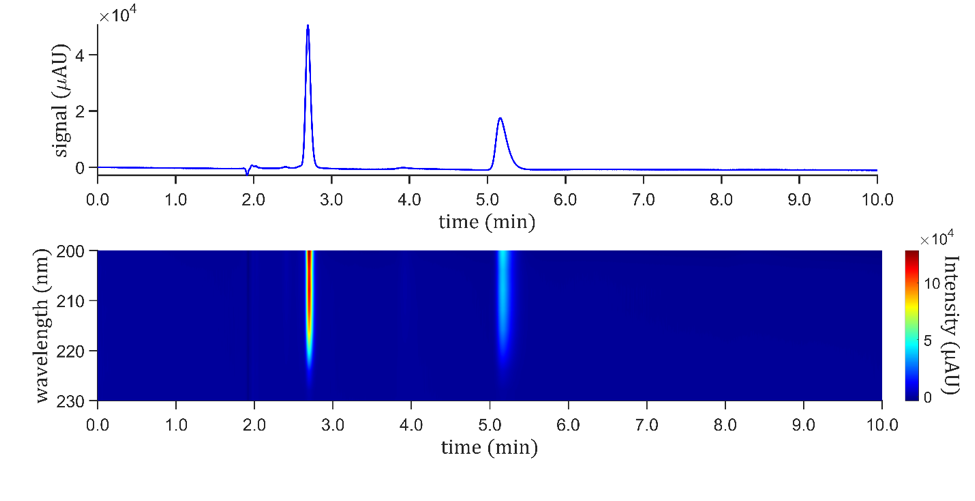
Porous graphitic carbon (PGC, 5 µm) is a stationary phase characterized by its extreme hydrophobicity, consisting of graphitized carbon sheets. Its flat carbon surface offers remarkable shape selectivity for adsorbed molecules, leading to exceptionally high selectivity between the S– and R-isomers, a feature not found in silica-based columns. Therefore, PGC is suitable for applications requiring a broad separation window between structural isomers, as in preparative work. As illustrated in Figure 3, S-amygdalin is eluted before the R-isomer, achieving an impressive resolution of 12 on a 150 mm x 4.6 mm i.d. column in 6 min. The peak shapes and efficiencies of the two isomers differ, with the second peak having a lower plate count, indicating that the naturally occurring R-amygdalin interacts strongly with the carbon surface, as evidenced by the plate count (7700 vs. 5600) plates for the S– and R-isomers, respectively. No additives were required in the acetonitrile-water eluent. As expected from the literature, the area ratio of the first to the second peak is 56.1 to 43.9 % [3].
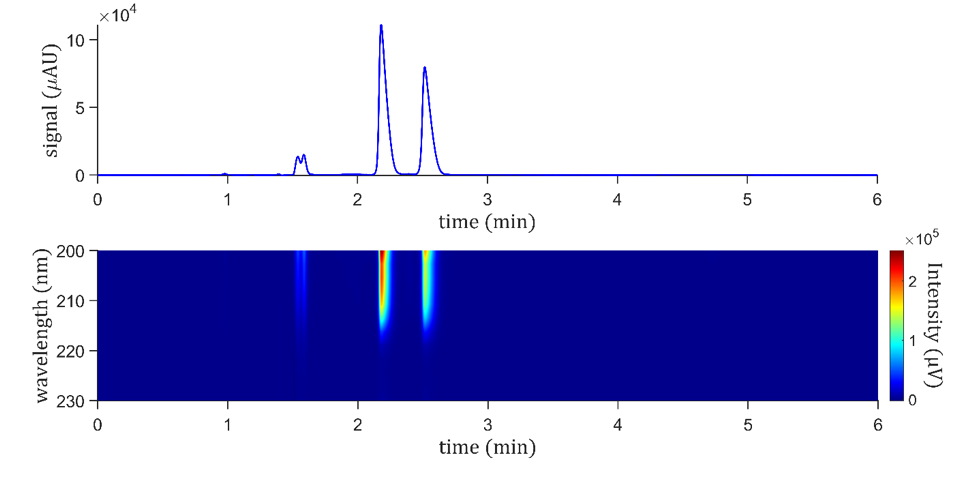
For rapid screening of chiral analytes, 2.7 µm superficially porous particles are suitable, which are bonded to chiral selectors. Figure 4 shows the separation of amygdalin epimers using (R, S)-hydroxypropyl-modified β-cyclodextrin chiral (CDShell-RSP) column. This column chemistry is very shape-selective in the reverse-phase mode for amygdalin using water and acetonitrile without additives. The column is 150 mm x 3 mm i.d. column. As illustrated in Figure 4, S-amygdalin is eluted before the R-isomer, like Hypercarb, achieving a good resolution of 2.7. Generally speaking, the chiral selector operates through the inclusion-complex formation, and an extended capability for hydrogen bonding, providing wide-ranging chiral and shape selectivity suitable for chiral screening, and it is particularly advantageous for basic and neutral compounds. This chiral column can rapidly separate the amygdalin epimers, achieving baseline resolution using an isocratic mobile phase composed of methanol and water, providing a plate count of 5700 (first peak) and 6140 (second peak) comparable to the porous graphitic column. The area ratio of the two major peaks (S– and R– amygdalin) is 55.5% to 44.5%. Both mobile phases, for the Hypercarb and CDShell-RSP, are also mass spectrometer friendly.
Real Sample Analysis
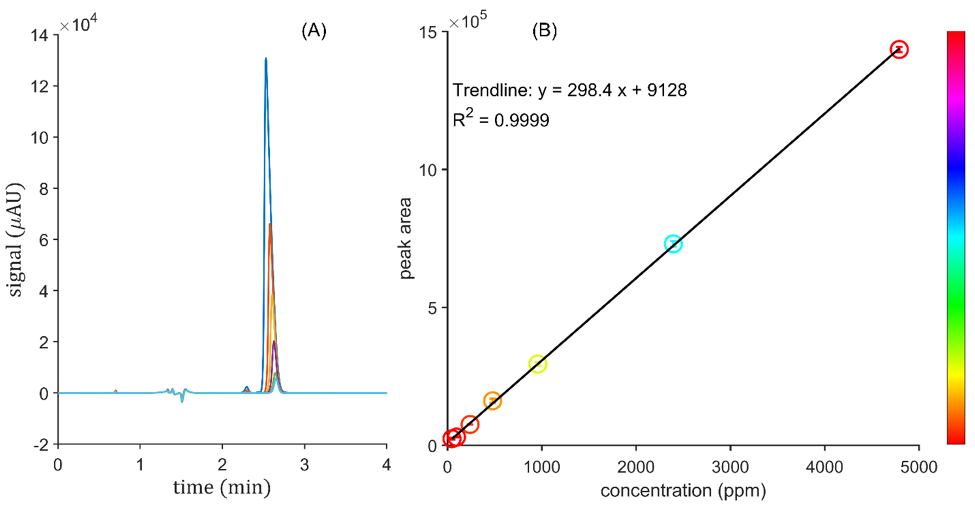
As noted earlier, natural amygdalin is often available as a supplement in capsule form and sometimes as an injectable solution. In this application note, a food supplement (claiming to be “vitamin B17”) was analyzed. These capsules were filled with a white powder, and the main ingredient is listed as being sourced from 98% pure amygdalin obtained from bitter apricot kernels. The calibration curve was prepared over two orders of magnitude in concentration (~ 48 to 4800 ppm). The linearity and correlation coefficient were excellent (R² = 0.9999) on JASCO’s PDA detector (Figure 5). The standards were made in 0.1% v/v acetic acid solution, to prevent epimerization and degradation, in 1 mL glass volumetric flasks [3]. The sample concentration was found to be 90.2% pure natural amygdalin by weight. The identity of any inert material in the capsule could not be ascertained.
Conclusion
Amygdalin, a cyanogenic glycoside predominantly found in the seeds and kernels of plants within the rose family, has played a substantial role in traditional Eastern medicine for various ailments without solid, clinically proven efficacy. Improper storage of amygdalin or exposure to slightly neutral or alkaline conditions can lead to the rapid epimerization of natural amygdalin to neoamygdalin, whose biological activity might differ from that of natural amygdalin. Two rapid analytical methods were proposed to separate and quantify natural amygdalin from its unnatural isomer using JASCO’s RHPLC and shape-selective columns. This application note can be extended to other cyanogenic glycosides, their epimers, and related compounds using simple reversed-phase eluents.
Required Products and Software
ChromNAV 2
References
- Dmitry V. Yashunsky, Ekaterina V. Kulakovskaya, Tatiana V. Kulakovskaya, Olga S. Zhukova, Mikhail V. Kiselevskiy, Nikolay E. Nifantiev, Synthesis and Biological Evaluation of Cyanogenic Glycosides, Journal of Carbohydrate Chemistry, 2015, 34, 460-474, DOI: 10.1080/07328303.2015.1105249
- Zuoqing Song. Xiaohong Xu, Advanced research on anti-tumor effects of amygdalin. Journal of Cancer Research and Therapeutics, 2014, 10 (Suppl 1), p C3-C7, DOI: 10.4103/0973-1482.139743
- Farooq Wahab, Zachary S. Breitbach, Daniel W. Armstrong, Rick Strattan, and Alain Berthod, Problems and Pitfalls in the Analysis of Amygdalin and Its Epimer, Journal of Agricultural and Food Chemistry, 2015, 63, 8966-8973, DOI: 10.1021/acs.jafc.5b03120.
- G. Wirthensohn, W. L. Chin, T. K. Franks, G. Baldock, C. M. Ford & M. Sedgley, Characterising the flavour phenotypes of almond (Prunus dulcisMill.) kernels, The Journal of Horticultural Science and Biotechnology, 2008, 83, 462-468, DOI: 10.1080/14620316.2008.11512407
REPORT PREPARED BY
(Experimentation/Investigation/ Figures): Ryan Jacob Burk
(Report writing & Supervision): M. Farooq Wahab and Daniel W. Armstrong
University of Texas at Arlington, USA

Chromatographic Analysis of Amygdalin Isomers
Introduction
Cyanogenic glycosides belong to a broad category of plant toxins [1]. The defining feature of these potentially toxic compounds is their ability to produce free hydrogen cyanide, a process called cyanogenesis. The generation of HCN is believed to function as a defense strategy for plants against fungal infections and predation. Since humans consume plants and their fruits and seeds, we can encounter these toxins inadvertently. These compounds appear in various structural forms across the plant world. Amygdalin is the most common cyanogenic glycoside found in seeds and kernels of the Rosaceae (rose family). Specifically, amygdalin, a primary constituent derived from bitter almonds, peaches, apricots, or cherry pits, has been a significant component of traditional Eastern medicine and has been the subject of research for almost two centuries [2]. When these seeds or nuts are eaten, amygdalin comes in contact with hydrolytic enzymes that first produce prunasin (D-mandelonitrile-β-D-glucoside) and glucose and, in a second step, prunasin can be converted into benzaldehyde and hydrogen cyanide, the mixture of these chemicals producing the pleasant almond-like aroma [3].

It is important to quantify the amount of HCN precursor, amygdalin, present in food and supplements. Since amygdalin is naturally present in several food items and is currently being marketed online as a nutritional supplement under the questionable designation of Vitamin B17, it is important to be able to monitor it using reliable analytical chromatography. In Figure 1, the structures of amygdalin (I) and its epimer (II) are shown. The compound has eleven stereogenic centers, ten of which are contained in the two glucopyranose moieties and are not easily isomerized. The remaining stereogenic center (i.e., the carbon atom with the phenyl and nitrile substituents) is susceptible to inversion, which can lead to the formation of neoamygdalin. Naturally occurring amygdalin exhibits the (R)-configuration at the phenyl substituted stereogenic center. This chiral center isomerizes in slightly basic conditions or neutral aqueous solutions, yielding the (S)-epimer (II). While the plant-extracted isomer of amygdalin primarily exists as the (R)-epimer, improper storage by companies can rapidly cause interconversion and even decomposition to other related compounds [4]. Amygdalin also shows an interesting behavior: its epimerization rate in aqueous solution depends on the glassware in which the solution is stored [3]. Some scientific inconsistencies are found in published analytical methods that often ignore the problem of amygdalin epimerization in glassware [3]. We establish reliable analytical methods using porous graphitic carbon and a cyclodextrin-based chiral stationary phase in the reversed phase mode to separate the two amygdalin epimers in less than six minutes using JASCO’s rapid HPLC or RHPLC with PDA detection.
Experimental
The JASCO RHPLC is equipped with a degasser, binary pump and an active mixer (PU-4180), autosampler (AS-4150) with a 5 µL sample loop, column oven (CO-4062), and photodiode array detector, PDA, (MD 4010). Columns: Hypercarb from Thermo Scientific, 150 mm x 4.6 mm i.d. column packed with 5 µm fully porous graphitic carbon, CDShell-RSP column (AZYP, LLC, Arlington, Texas), 150×3 mm i.d. packed with 2.7 µm superficially porous particles (SPP). Chemicals: HPLC grade acetonitrile, methanol, deionized water (18 MΩ-cm), and amygdalin standard (CAS No. 29883-15-6) were obtained from Millipore Sigma (USA). The amygdalin is extracted from apricot kernels, and the source code is SLCK7401. Mobile phase compositions refer to volume-by-volume (v/v) compositions. A single pump was used after manually mixing the solvents. The calibration curve solutions were prepared in 1 mL volumetric flasks in deionized water with a trace of acetic acid to prevent epimerization.
Results
Epimerization of Amygdalin

Given the fact that the natural amygdalin in aqueous solutions can rapidly epimerize to neoamygdalin (II), columns must be screened for epimeric selectivity. The epimerization of (I) can be conveniently carried out by adding aqueous ammonia. A typical approach could be measuring 0.05 g of amygdalin in 5 mL of deionized water in a clean glass vial, adding 10 µL of NH3, and allowing about 20 mins to epimerize at room temperature. This process is shown in Figure 2. Under equilibrium conditions, neoamygdalin dominates slightly, typically around 57% to 43%.
Separation of Neoamygdalin and Amygdalin
In the early analytical methods, amygdalin epimers were differentiated through gas chromatography (GC) using trifluoroacetylated derivatives. Subsequently, capillary electrophoresis was employed to distinguish the epimers within 15 minutes, showing commendable selectivity with a cyclodextrin chiral additive in the run buffer. Despite these methods, in most research, liquid chromatography (LC) with a C-18 phase appears to have been the preferred approach, even though it led to overlapping of adjacent peaks for the R– and S– forms of amygdalin.

Porous graphitic carbon (PGC, 5 µm) is a stationary phase characterized by its extreme hydrophobicity, consisting of graphitized carbon sheets. Its flat carbon surface offers remarkable shape selectivity for adsorbed molecules, leading to exceptionally high selectivity between the S– and R-isomers, a feature not found in silica-based columns. Therefore, PGC is suitable for applications requiring a broad separation window between structural isomers, as in preparative work. As illustrated in Figure 3, S-amygdalin is eluted before the R-isomer, achieving an impressive resolution of 12 on a 150 mm x 4.6 mm i.d. column in 6 min. The peak shapes and efficiencies of the two isomers differ, with the second peak having a lower plate count, indicating that the naturally occurring R-amygdalin interacts strongly with the carbon surface, as evidenced by the plate count (7700 vs. 5600) plates for the S– and R-isomers, respectively. No additives were required in the acetonitrile-water eluent. As expected from the literature, the area ratio of the first to the second peak is 56.1 to 43.9 % [3].

For rapid screening of chiral analytes, 2.7 µm superficially porous particles are suitable, which are bonded to chiral selectors. Figure 4 shows the separation of amygdalin epimers using (R, S)-hydroxypropyl-modified β-cyclodextrin chiral (CDShell-RSP) column. This column chemistry is very shape-selective in the reverse-phase mode for amygdalin using water and acetonitrile without additives. The column is 150 mm x 3 mm i.d. column. As illustrated in Figure 4, S-amygdalin is eluted before the R-isomer, like Hypercarb, achieving a good resolution of 2.7. Generally speaking, the chiral selector operates through the inclusion-complex formation, and an extended capability for hydrogen bonding, providing wide-ranging chiral and shape selectivity suitable for chiral screening, and it is particularly advantageous for basic and neutral compounds. This chiral column can rapidly separate the amygdalin epimers, achieving baseline resolution using an isocratic mobile phase composed of methanol and water, providing a plate count of 5700 (first peak) and 6140 (second peak) comparable to the porous graphitic column. The area ratio of the two major peaks (S– and R– amygdalin) is 55.5% to 44.5%. Both mobile phases, for the Hypercarb and CDShell-RSP, are also mass spectrometer friendly.

Real Sample Analysis
As noted earlier, natural amygdalin is often available as a supplement in capsule form and sometimes as an injectable solution. In this application note, a food supplement (claiming to be “vitamin B17”) was analyzed. These capsules were filled with a white powder, and the main ingredient is listed as being sourced from 98% pure amygdalin obtained from bitter apricot kernels. The calibration curve was prepared over two orders of magnitude in concentration (~ 48 to 4800 ppm). The linearity and correlation coefficient were excellent (R² = 0.9999) on JASCO’s PDA detector (Figure 5). The standards were made in 0.1% v/v acetic acid solution, to prevent epimerization and degradation, in 1 mL glass volumetric flasks [3]. The sample concentration was found to be 90.2% pure natural amygdalin by weight. The identity of any inert material in the capsule could not be ascertained.
Conclusion
Amygdalin, a cyanogenic glycoside predominantly found in the seeds and kernels of plants within the rose family, has played a substantial role in traditional Eastern medicine for various ailments without solid, clinically proven efficacy. Improper storage of amygdalin or exposure to slightly neutral or alkaline conditions can lead to the rapid epimerization of natural amygdalin to neoamygdalin, whose biological activity might differ from that of natural amygdalin. Two rapid analytical methods were proposed to separate and quantify natural amygdalin from its unnatural isomer using JASCO’s RHPLC and shape-selective columns. This application note can be extended to other cyanogenic glycosides, their epimers, and related compounds using simple reversed-phase eluents.
Keywords
HPLC, Chromatography, Amygdalin, Neoamygdalin, Cyanogenic Glycosides, Rosaceae, Seeds, Chiral, Epimer, Vitamin B17
Required Products and Software
ChromNAV 2
References
- Dmitry V. Yashunsky, Ekaterina V. Kulakovskaya, Tatiana V. Kulakovskaya, Olga S. Zhukova, Mikhail V. Kiselevskiy, Nikolay E. Nifantiev, Synthesis and Biological Evaluation of Cyanogenic Glycosides, Journal of Carbohydrate Chemistry, 2015, 34, 460-474, DOI: 10.1080/07328303.2015.1105249
- Zuoqing Song. Xiaohong Xu, Advanced research on anti-tumor effects of amygdalin. Journal of Cancer Research and Therapeutics, 2014, 10 (Suppl 1), p C3-C7, DOI: 10.4103/0973-1482.139743
- Farooq Wahab, Zachary S. Breitbach, Daniel W. Armstrong, Rick Strattan, and Alain Berthod, Problems and Pitfalls in the Analysis of Amygdalin and Its Epimer, Journal of Agricultural and Food Chemistry, 2015, 63, 8966-8973, DOI: 10.1021/acs.jafc.5b03120.
- G. Wirthensohn, W. L. Chin, T. K. Franks, G. Baldock, C. M. Ford & M. Sedgley, Characterising the flavour phenotypes of almond (Prunus dulcisMill.) kernels, The Journal of Horticultural Science and Biotechnology, 2008, 83, 462-468, DOI: 10.1080/14620316.2008.11512407
REPORT PREPARED BY
(Experimentation/Investigation/ Figures): Ryan Jacob Burk
(Report writing & Supervision): M. Farooq Wahab and Daniel W. Armstrong
University of Texas at Arlington, USA

 Download This Application
Download This Application