CPL Spectrum Measurement of Green Fluorescent Protein (GFP)
January 5, 2024
Introduction

Circularly polarized luminescence (CPL) measures the difference in the emission intensities of left- and right-handed circularly polarized light of chiral compounds. Unlike CD, CPL provides information on the excited state properties of chiral molecules.
Green fluorescence protein (GFP) is a protein that emits green fluorescence when exposed to ultraviolet light and has been shown to have chiroptical properties1. This application note illustrates the CPL and CD spectra of GFP obtained with the CPL-300 and J-1500, respectively.
Experimental
| Measurement Conditions CPL-300 Circular Polarized Luminescence |
|||
| Excitation Wavelength | 399 nm | Excitation Bandwidth | 12 nm |
| Data Interval | 1 nm | Response | 16 seconds |
| Emission Bandwidth | 8 nm | Scan Speed | 10 nm/min |
| Accumulations | 36 | Path length | 10 mm |
| Measurement Conditions J-1500 Circular Dichroism Spectrophotometer |
|||
| Data Pitch | 0.2 nm | D.I.T. | 1 sec |
| Bandwidth | 1 nm | Scan Speed | 200 nm/min |
| Accumulations | 16 | Path Length | 20 mm |
Keywords
210-CD-0030, J-1500, Circular dichroism, CPL-300, Circularly polarized luminescence, Protein structure, Fluorescence, Biochemistry
Results
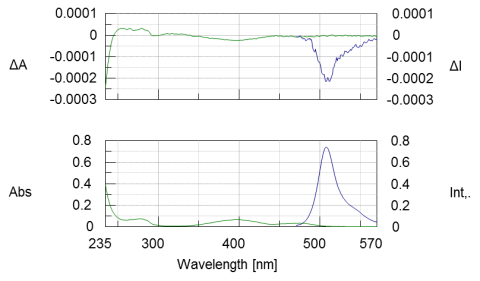
CD and absorption spectra (Figure 1, green) of a 0.03 mg/mL GFP solution was measured in the near-UV and visible regions. The GFP chromophore is observed in the visible region at 400 nm and the absorption bands due to the aromatic amino acid residues are observed in the near-UV region between 250 and 300 nm. The CPL and fluorescence spectra were also obtained and show in blue in Figure 1. The characteristic fluorescent and CPL spectra of GFP are observed between 470-570 nm.
References
1. Hiromasa Goto, Isao Sawada, and Nobuhiko Nomura, International Journal of Polymeric Materials, 2010, 59, 786-792.
Featured Products:

CPL Spectrum Measurement of Green Fluorescent Protein (GFP)
Introduction

Circularly polarized luminescence (CPL) measures the difference in the emission intensities of left- and right-handed circularly polarized light of chiral compounds. Unlike CD, CPL provides information on the excited state properties of chiral molecules.
Green fluorescence protein (GFP) is a protein that emits green fluorescence when exposed to ultraviolet light and has been shown to have chiroptical properties1. This application note illustrates the CPL and CD spectra of GFP obtained with the CPL-300 and J-1500, respectively.
Experimental
| Measurement Conditions CPL-300 Circular Polarized Luminescence |
|||
| Excitation Wavelength | 399 nm | Excitation Bandwidth | 12 nm |
| Data Interval | 1 nm | Response | 16 seconds |
| Emission Bandwidth | 8 nm | Scan Speed | 10 nm/min |
| Accumulations | 36 | Path length | 10 mm |
| Measurement Conditions J-1500 Circular Dichroism Spectrophotometer |
|||
| Data Pitch | 0.2 nm | D.I.T. | 1 sec |
| Bandwidth | 1 nm | Scan Speed | 200 nm/min |
| Accumulations | 16 | Path Length | 20 mm |
Keywords
210-CD-0030, J-1500, Circular dichroism, CPL-300, Circularly polarized luminescence, Protein structure, Fluorescence, Biochemistry
Results
CD and absorption spectra (Figure 1, green) of a 0.03 mg/mL GFP solution was measured in the near-UV and visible regions. The GFP chromophore is observed in the visible region at 400 nm and the absorption bands due to the aromatic amino acid residues are observed in the near-UV region between 250 and 300 nm. The CPL and fluorescence spectra were also obtained and show in blue in Figure 1. The characteristic fluorescent and CPL spectra of GFP are observed between 470-570 nm.

References
1. Hiromasa Goto, Isao Sawada, and Nobuhiko Nomura, International Journal of Polymeric Materials, 2010, 59, 786-792.

 Download This Application
Download This Application