Enantiomeric Analysis of Nicotine in E-Liquids by Subcritical Fluid Chromatography
April 16, 2024Introduction
(S)-nicotine is a naturally occurring neuroactive alkaloid found in the Nicotiana tabacum plant, commonly known as tobacco. Nicotine’s molecular structure (Figure 1) is unique as it contains both a five-membered (pyrrolidine) and a six membered (pyridine) nitrogen heterocycle, with one chiral center. Naturally occurring nicotine is commercially referred to as “tobacco derived nicotine” (TDN) in Vape and E-liquid products and exists in an excess (>90%) of the (S)-enantiomer. TDN products have been subject to stringent regulation by the United States Food and Drug Agency (US FDA) since the passing of the “Family Smoking Prevention and Tobacco Control Act” in 2009 and the “Deeming Rule” in 2016.1 However, racemic nicotine produced synthetically is commercially known as “tobacco free nicotine” (TFN) and was not subject to the same regulation since it is not a tobacco product.2 This led to the incorporation of this racemic nicotine into many commercial products to avoid regulation even though the biological activity and toxicology of (R)-nicotine are not very well known.2 In 2022, the US Congress passed a federal law allowing the FDA to regulate TFN products,1 however, these racemic products were already in circulation. Therefore, a method to analyze nicotine’s enantiomeric purity must be developed for regulatory and quality assurance purposes.
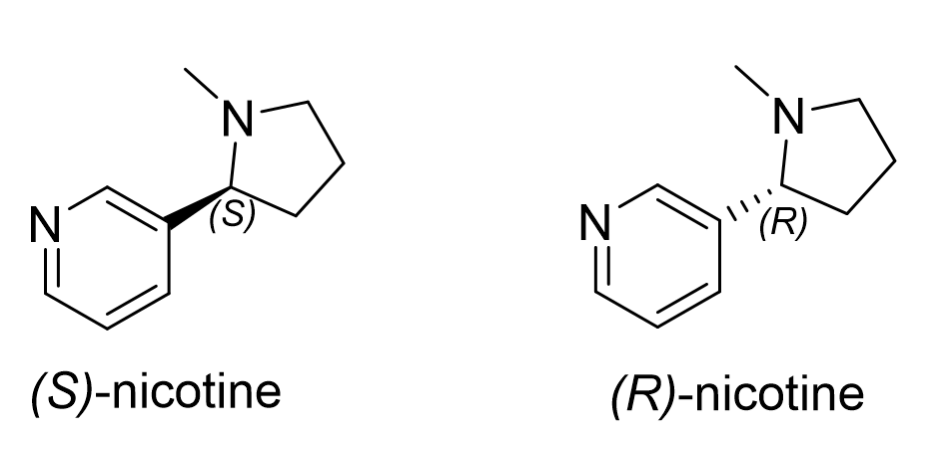
The geometric configuration of nicotine (Figure 1) makes its enantioseparation notoriously difficult, with the earliest gas and liquid chromatographic methods (the 1980s onwards) requiring several hours to resolve the enantiomers poorly.2 In 2017, this separation was revolutionized when a new chiral stationary phase (CSP) composed of modified macrocyclic glycopeptides bonded to superficially porous particles (SPPs) was developed by AZYP, LLC and later commercialized as “NicoShell.” This CSP can directly separate nicotine’s enantiomers in just a few minutes with excellent resolution using the polar ionic mode of liquid chromatography.3 NicoShell was then used to complete the first sub/supercritical fluid chromatographic separation of nicotine’s enantiomers in 2019.4 Here, we demonstrate a method to analyze nicotine’s concentration and enantiomeric purity in E-liquids simultaneously. The method provides low retention for the other components present in E-liquids, eluting them near the dead volume, which allows for analysis of these commercial products using only “dilute and shoot” type sample preparation. The low viscosity and high diffusivity of the carbon-dioxide containing mobile phase allow for high flow rates (4 mL/min) and, subsequently, rapid analysis (< 3 min) suitable for a high-throughput environment using sub/supercritical fluid chromatographs (SFC).
Experimental
All separations conducted in this report were done using the JASCO 2000 series SFC. This instrument is equipped with two intelligent semi-preparative pumps (PU-2086), an autosampler (AS-2059-SFC) with a 5 µL loop, a column oven (CO-2060), a UV detector (UV-2075), and a back pressure regulator (BP-2080). The NicoShell modified macrocyclic glycopeptide column was acquired from AZYP, LLC (Arlington, TX, USA) in the dimensions of 100 mm x 4.6 mm (i.d.) with 2.7 µm SPPs. The organic modifier for this method contains LC-MS grade methanol (MeOH) and triethylamine (TEA) in a 100 to 0.2 volumetric ratio. The mobile phase for all separations contained 50/50 modifier/CO2 at a flow rate of 4 mL/min, a column temperature of 30º C, a back pressure (BP) of 8 MPa, and an injection volume of 5 µL. E-liquids were acquired from a local tobacco store and diluted in a 30 to 1 volumetric ratio with MeOH. Racemic and (S)-nicotine standards were purchased from Sigma-Aldrich. Solutions from these standards were prepared at 0.5 mg/mL concentration in MeOH.
Keywords
Tobacco, Nicotine, SFC, Fast LC, E-Liquids, Enantiomers
Results
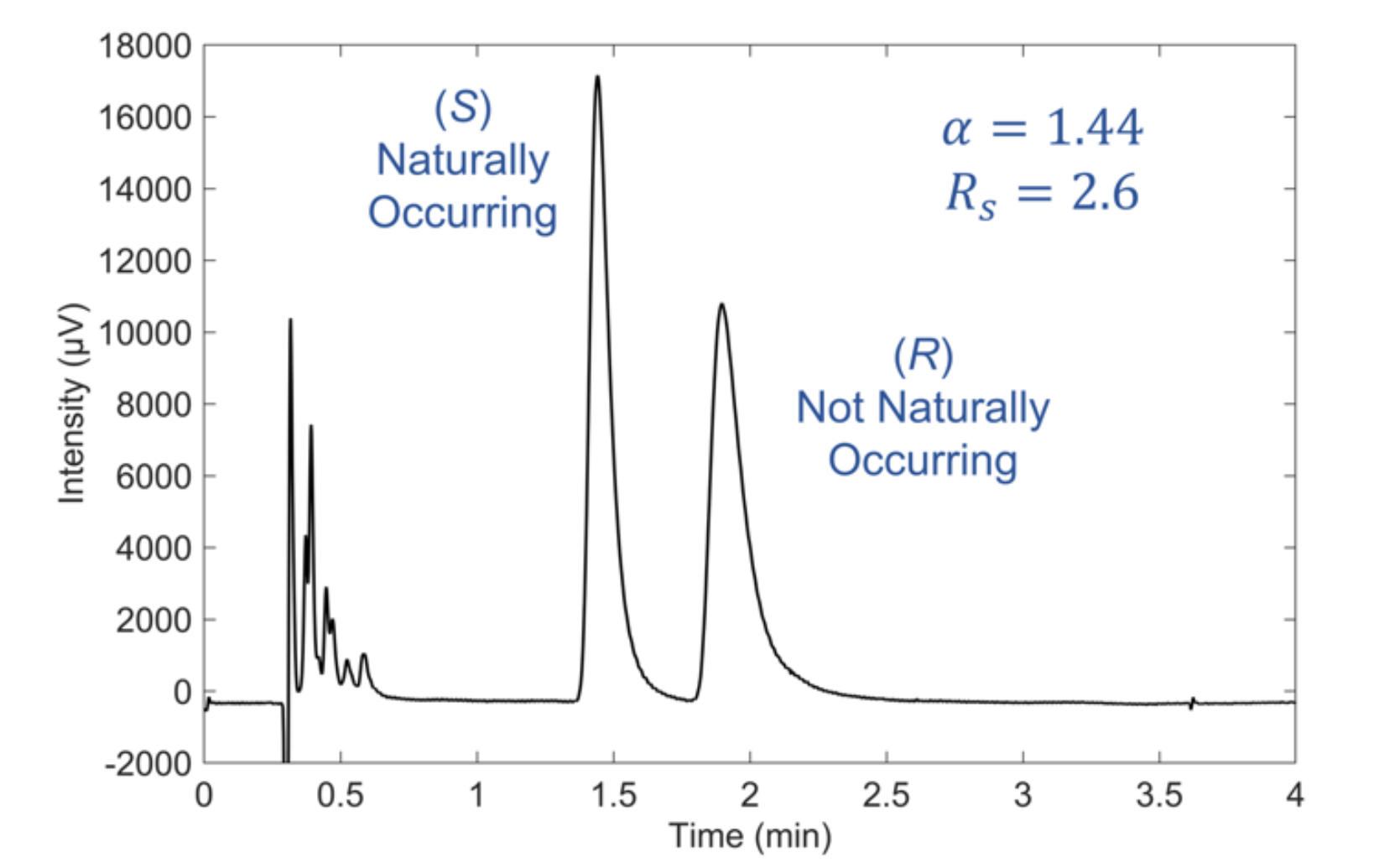
The separation of racemic nicotine using the developed SFC method is shown in Figure 2. The entire separation is conducted in less than 2.5 minutes, with satisfactory enantioselectivity (α = 1.44) and baseline resolution (Rs = 2.6). This method was used for the remainder of this report.
Enantiomeric Analysis of Commercial E-Liquids
E-liquids provide an extremely involved sample matrix when analyzing nicotine due to the large number of components present and the high viscosity of the sample caused by the majority of the volume being glycerin, glycol, or related compounds. For reference, a recent study using LC paired with high-resolution mass spectrometry found that an E-liquid can have a multitude of distinguishable components.5 The structures of these components were not assigned implying their effects on human toxicology are also unknown. The NicoShell has a semisynthetic macrocyclic glycopeptide chiral selector, which has the unique ability to retain nicotine while eluting the majority of the E-liquid components (at least those detectable by UV at the desired wavelength) near the dead volume of the column. This allows for the convenient isocratic analysis of nicotine, be it for enantiomeric purity or just for quantification, without extending run times to elute the remainder of compounds. This special stationary phase chemistry also allows for the analysis of nicotine without needing any sample preparation except dilution.
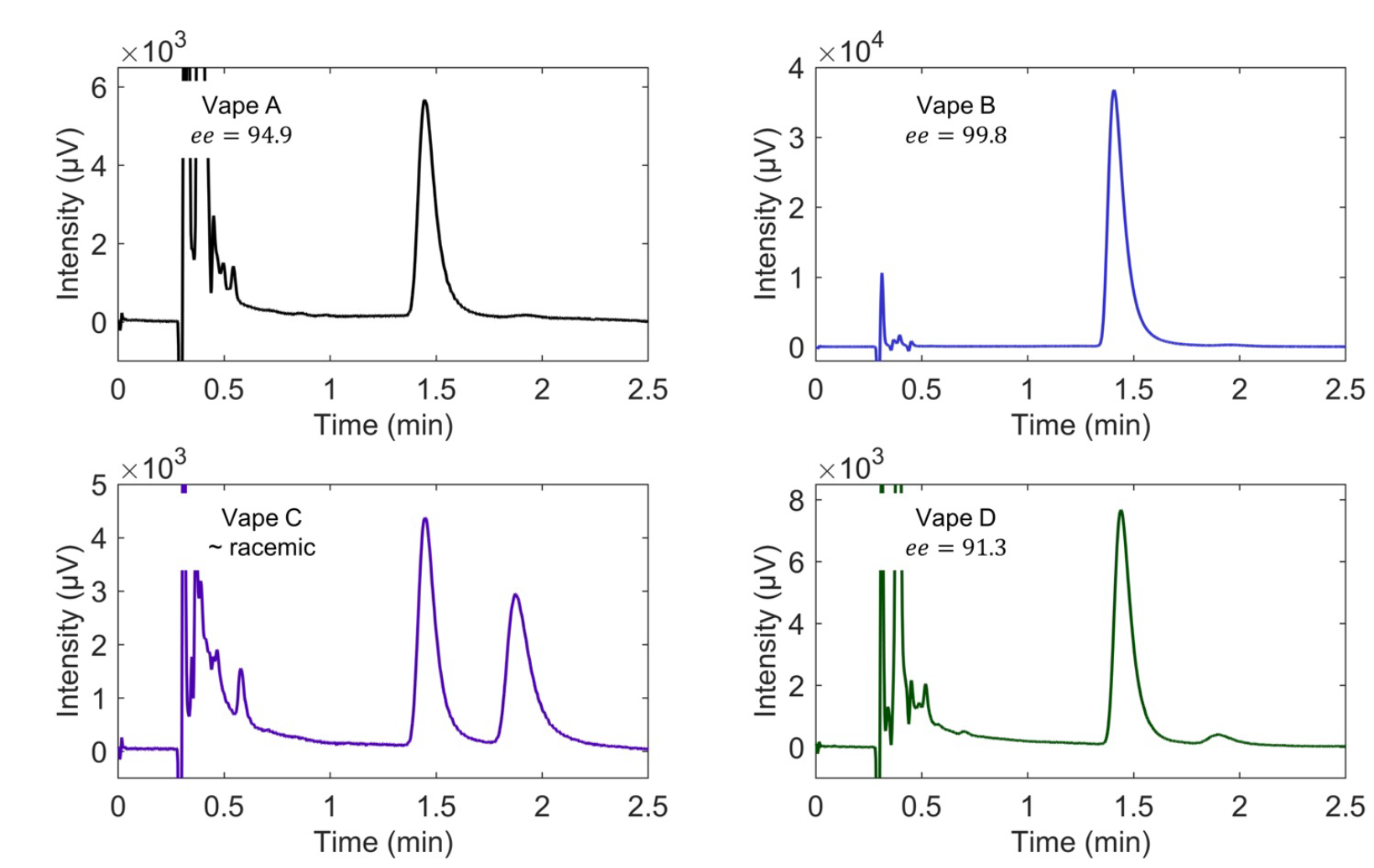
Figure 3 shows an example analysis of four commercial E-liquids using the developed method on JASCO SFC. In all cases, it can be seen that a large number of compounds elute around 0.5 minutes. Vapes A, B, and D contain one prominent peak for the (S)-enantiomer and only a small peak for the (R)-enantiomer, insinuating that these are tobacco-derived products. The enantiomeric excess (ee = ([R] – [S])/([R] + [S])) of the (S)-enantiomer ranges from 91.3 to 99.8, showing that not all sources of TDN have the same enantiomeric composition. Vape C, in this example, is the only product that contains nearly racemic nicotine. This confirms that the sample is from a TFN source. By analyzing these samples, it can be readily seen what samples are made with TFN or TDN sources in just a few minutes. This same data can be used to quantify the nicotine amount in the samples, as discussed below.
Quantification of Nicotine in Commercial E-Liquids
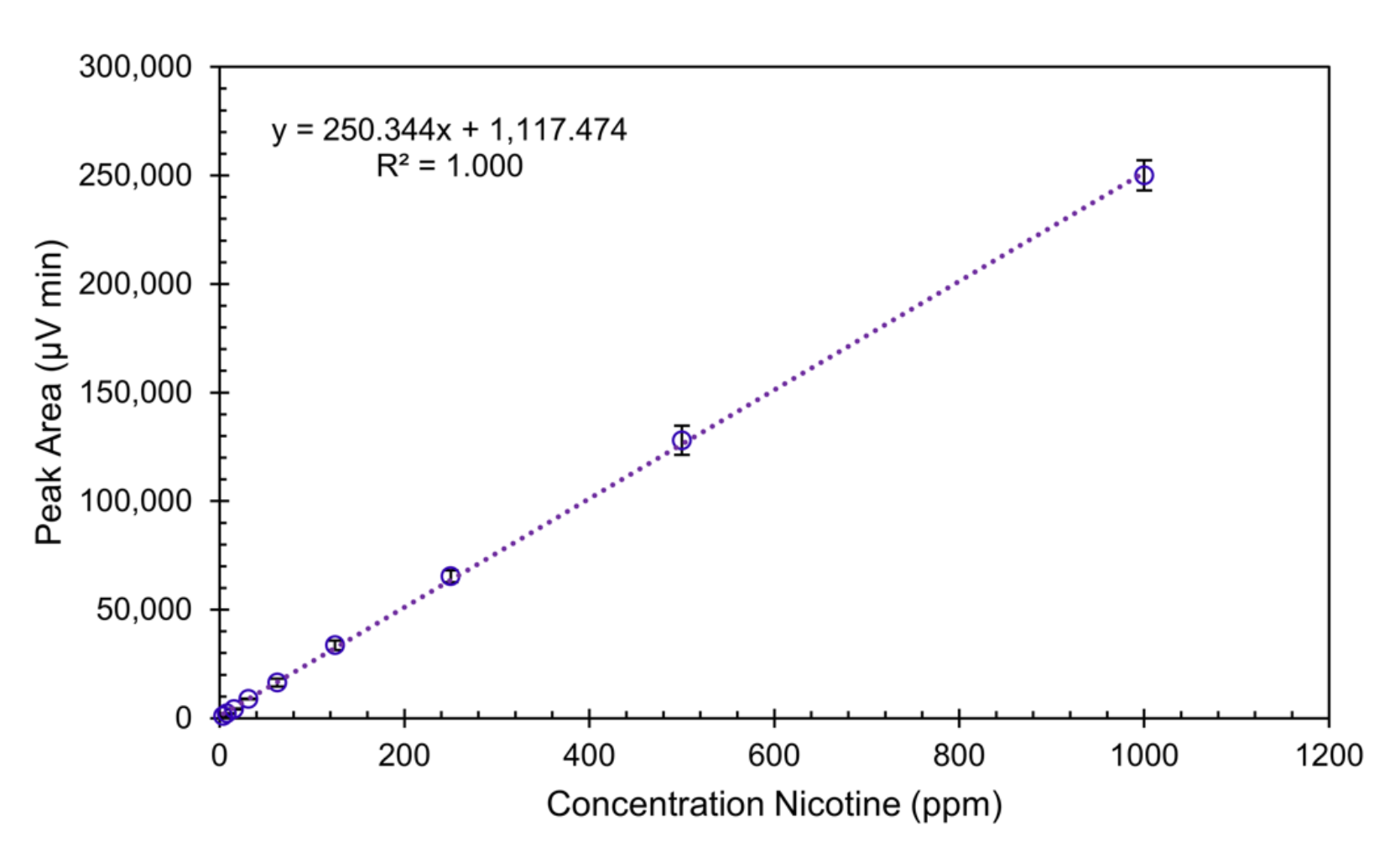
Enantiomers are optical isomers and must absorb plane polarized light identically. Therefore, one calibration curve can be made to approximate the instrument’s response (area) as a function of concentration for both enantiomers. This allows users to calibrate the chromatograph for nicotine using the readily available (S)-enantiomer if they desire. Figure 4 shows the calibration curve for (S)-nicotine with concentrations ranging from 3.90 ppm to 2,000 ppm. This calibration curve shows that the method has excellent linearity over nearly three orders of magnitude. From this calibration curve, the concentration of both nicotine enantiomers can be calculated independently and summed to represent the total nicotine concentration.
Conclusion
The analysis of enantiomeric composition in tobacco-free nicotine products is critical for evaluating their health impacts and for current and future regulatory compliance of tobacco related products. This application note has demonstrated that subcritical fluid chromatography (SFC) is an effective technique for separating nicotine enantiomers rapidly. The utilization of CO2 and MeOH as a mobile phase in combination with the NicoShell column has proven to be particularly effective, achieving enantioseparation in under three minutes. The precision and speed of this method will allow quality control and ensure that the enantiomeric purity of nicotine in these products is accurately monitored during manufacture. This application note will also allow researchers to study the correlation of enantiomeric ratios of nicotine isomers with pharmacological effects. JASCO’s semi-preparative SFC is also an excellent tool for conducting cost-effective preparative separation of pure (R)-nicotine, which is not widely available as an enantiopure product.
References
Experimentation, figures, report writing: Bailey C. Glass and Troy T. Handlovic
Review, conceptualization, & supervision: M. Farooq Wahab and Daniel W. Armstrong
(1) Requirements for Products Made with Non-Tobacco Nicotine Take Effect April. US Food and Drug Administration. https://www.fda.gov/tobacco-products/ctp-newsroom/requirements-products-made-non-tobacco-nicotine-take-effect-april-14 (accessed 2024-01-17).
(2) Salam, S.; El-Hajj Moussa, F.; El-Hage, R.; El-Hellani, A.; Aoun Saliba, N. A Systematic Review of Analytical Methods for the Separation of Nicotine Enantiomers and Evaluation of Nicotine Sources. Chem Res Toxicol 2023, 36 (3), 334–341. https://doi.org/10.1021/acs.chemrestox.2c00310.
(3) Hellinghausen, G.; Lee, J. T.; Weatherly, C. A.; Lopez, D. A.; Armstrong, D. W. Evaluation of Nicotine in Tobacco-Free-Nicotine Commercial Products. Drug Test Anal 2017, 9 (6), 944–948. https://doi.org/10.1002/dta.2145.
(4) Roy, D.; Armstrong, D. W. Fast Super/Subcritical Fluid Chromatographic Enantioseparations on Superficially Porous Particles Bonded with Broad Selectivity Chiral Selectors Relative to Fully Porous Particles. J Chromatogr A 2019, 1605. https://doi.org/10.1016/j.chroma.2019.06.060.
(5) Tehrani, M. W.; Newmeyer, M. N.; Rule, A. M.; Prasse, C. Characterizing the Chemical Landscape in Commercial E-Cigarette Liquids and Aerosols by Liquid Chromatography–High-Resolution Mass Spectrometry. Chem Res Toxicol 2021, 34 (10), 2216–2226. https://doi.org/10.1021/acs.chemrestox.1c00253.

Enantiomeric Analysis of Nicotine in E-Liquids by Subcritical Fluid Chromatography
Introduction
(S)-nicotine is a naturally occurring neuroactive alkaloid found in the Nicotiana tabacum plant, commonly known as tobacco. Nicotine’s molecular structure (Figure 1) is unique as it contains both a five-membered (pyrrolidine) and a six membered (pyridine) nitrogen heterocycle, with one chiral center. Naturally occurring nicotine is commercially referred to as “tobacco derived nicotine” (TDN) in Vape and E-liquid products and exists in an excess (>90%) of the (S)-enantiomer. TDN products have been subject to stringent regulation by the United States Food and Drug Agency (US FDA) since the passing of the “Family Smoking Prevention and Tobacco Control Act” in 2009 and the “Deeming Rule” in 2016.1 However, racemic nicotine produced synthetically is commercially known as “tobacco free nicotine” (TFN) and was not subject to the same regulation since it is not a tobacco product.2 This led to the incorporation of this racemic nicotine into many commercial products to avoid regulation even though the biological activity and toxicology of (R)-nicotine are not very well known.2 In 2022, the US Congress passed a federal law allowing the FDA to regulate TFN products,1 however, these racemic products were already in circulation. Therefore, a method to analyze nicotine’s enantiomeric purity must be developed for regulatory and quality assurance purposes.

The geometric configuration of nicotine (Figure 1) makes its enantioseparation notoriously difficult, with the earliest gas and liquid chromatographic methods (the 1980s onwards) requiring several hours to resolve the enantiomers poorly.2 In 2017, this separation was revolutionized when a new chiral stationary phase (CSP) composed of modified macrocyclic glycopeptides bonded to superficially porous particles (SPPs) was developed by AZYP, LLC and later commercialized as “NicoShell.” This CSP can directly separate nicotine’s enantiomers in just a few minutes with excellent resolution using the polar ionic mode of liquid chromatography.3 NicoShell was then used to complete the first sub/supercritical fluid chromatographic separation of nicotine’s enantiomers in 2019.4 Here, we demonstrate a method to analyze nicotine’s concentration and enantiomeric purity in E-liquids simultaneously. The method provides low retention for the other components present in E-liquids, eluting them near the dead volume, which allows for analysis of these commercial products using only “dilute and shoot” type sample preparation. The low viscosity and high diffusivity of the carbon-dioxide containing mobile phase allow for high flow rates (4 mL/min) and, subsequently, rapid analysis (< 3 min) suitable for a high-throughput environment using sub/supercritical fluid chromatographs (SFC).
Experimental
All separations conducted in this report were done using the JASCO 2000 series SFC. This instrument is equipped with two intelligent semi-preparative pumps (PU-2086), an autosampler (AS-2059-SFC) with a 5 µL loop, a column oven (CO-2060), a UV detector (UV-2075), and a back pressure regulator (BP-2080). The NicoShell modified macrocyclic glycopeptide column was acquired from AZYP, LLC (Arlington, TX, USA) in the dimensions of 100 mm x 4.6 mm (i.d.) with 2.7 µm SPPs. The organic modifier for this method contains LC-MS grade methanol (MeOH) and triethylamine (TEA) in a 100 to 0.2 volumetric ratio. The mobile phase for all separations contained 50/50 modifier/CO2 at a flow rate of 4 mL/min, a column temperature of 30º C, a back pressure (BP) of 8 MPa, and an injection volume of 5 µL. E-liquids were acquired from a local tobacco store and diluted in a 30 to 1 volumetric ratio with MeOH. Racemic and (S)-nicotine standards were purchased from Sigma-Aldrich. Solutions from these standards were prepared at 0.5 mg/mL concentration in MeOH.
Keywords
Tobacco, Nicotine, SFC, Fast LC, E-Liquids, Enantiomers
Results
The separation of racemic nicotine using the developed SFC method is shown in Figure 2. The entire separation is conducted in less than 2.5 minutes, with satisfactory enantioselectivity (α = 1.44) and baseline resolution (Rs = 2.6). This method was used for the remainder of this report.

Enantiomeric Analysis of Commercial E-Liquids
E-liquids provide an extremely involved sample matrix when analyzing nicotine due to the large number of components present and the high viscosity of the sample caused by the majority of the volume being glycerin, glycol, or related compounds. For reference, a recent study using LC paired with high-resolution mass spectrometry found that an E-liquid can have a multitude of distinguishable components.5 The structures of these components were not assigned implying their effects on human toxicology are also unknown. The NicoShell has a semisynthetic macrocyclic glycopeptide chiral selector, which has the unique ability to retain nicotine while eluting the majority of the E-liquid components (at least those detectable by UV at the desired wavelength) near the dead volume of the column. This allows for the convenient isocratic analysis of nicotine, be it for enantiomeric purity or just for quantification, without extending run times to elute the remainder of compounds. This special stationary phase chemistry also allows for the analysis of nicotine without needing any sample preparation except dilution.

Figure 3 shows an example analysis of four commercial E-liquids using the developed method on JASCO SFC. In all cases, it can be seen that a large number of compounds elute around 0.5 minutes. Vapes A, B, and D contain one prominent peak for the (S)-enantiomer and only a small peak for the (R)-enantiomer, insinuating that these are tobacco-derived products. The enantiomeric excess (ee = ([R] – [S])/([R] + [S])) of the (S)-enantiomer ranges from 91.3 to 99.8, showing that not all sources of TDN have the same enantiomeric composition. Vape C, in this example, is the only product that contains nearly racemic nicotine. This confirms that the sample is from a TFN source. By analyzing these samples, it can be readily seen what samples are made with TFN or TDN sources in just a few minutes. This same data can be used to quantify the nicotine amount in the samples, as discussed below.
Quantification of Nicotine in Commercial E-Liquids

Enantiomers are optical isomers and must absorb plane polarized light identically. Therefore, one calibration curve can be made to approximate the instrument’s response (area) as a function of concentration for both enantiomers. This allows users to calibrate the chromatograph for nicotine using the readily available (S)-enantiomer if they desire. Figure 4 shows the calibration curve for (S)-nicotine with concentrations ranging from 3.90 ppm to 2,000 ppm. This calibration curve shows that the method has excellent linearity over nearly three orders of magnitude. From this calibration curve, the concentration of both nicotine enantiomers can be calculated independently and summed to represent the total nicotine concentration.
Conclusion
The analysis of enantiomeric composition in tobacco-free nicotine products is critical for evaluating their health impacts and for current and future regulatory compliance of tobacco related products. This application note has demonstrated that subcritical fluid chromatography (SFC) is an effective technique for separating nicotine enantiomers rapidly. The utilization of CO2 and MeOH as a mobile phase in combination with the NicoShell column has proven to be particularly effective, achieving enantioseparation in under three minutes. The precision and speed of this method will allow quality control and ensure that the enantiomeric purity of nicotine in these products is accurately monitored during manufacture. This application note will also allow researchers to study the correlation of enantiomeric ratios of nicotine isomers with pharmacological effects. JASCO’s semi-preparative SFC is also an excellent tool for conducting cost-effective preparative separation of pure (R)-nicotine, which is not widely available as an enantiopure product.
References
Experimentation, figures, report writing: Bailey C. Glass and Troy T. Handlovic
Review, conceptualization, & supervision: M. Farooq Wahab and Daniel W. Armstrong
(1) Requirements for Products Made with Non-Tobacco Nicotine Take Effect April. US Food and Drug Administration. https://www.fda.gov/tobacco-products/ctp-newsroom/requirements-products-made-non-tobacco-nicotine-take-effect-april-14 (accessed 2024-01-17).
(2) Salam, S.; El-Hajj Moussa, F.; El-Hage, R.; El-Hellani, A.; Aoun Saliba, N. A Systematic Review of Analytical Methods for the Separation of Nicotine Enantiomers and Evaluation of Nicotine Sources. Chem Res Toxicol 2023, 36 (3), 334–341. https://doi.org/10.1021/acs.chemrestox.2c00310.
(3) Hellinghausen, G.; Lee, J. T.; Weatherly, C. A.; Lopez, D. A.; Armstrong, D. W. Evaluation of Nicotine in Tobacco-Free-Nicotine Commercial Products. Drug Test Anal 2017, 9 (6), 944–948. https://doi.org/10.1002/dta.2145.
(4) Roy, D.; Armstrong, D. W. Fast Super/Subcritical Fluid Chromatographic Enantioseparations on Superficially Porous Particles Bonded with Broad Selectivity Chiral Selectors Relative to Fully Porous Particles. J Chromatogr A 2019, 1605. https://doi.org/10.1016/j.chroma.2019.06.060.
(5) Tehrani, M. W.; Newmeyer, M. N.; Rule, A. M.; Prasse, C. Characterizing the Chemical Landscape in Commercial E-Cigarette Liquids and Aerosols by Liquid Chromatography–High-Resolution Mass Spectrometry. Chem Res Toxicol 2021, 34 (10), 2216–2226. https://doi.org/10.1021/acs.chemrestox.1c00253.

 Download This Application
Download This Application