Evaluation of Temperature and Pressure Parameters for Increased Resolution for the Separation of Alkanes and Aromatics Using SFC
August 15, 2022
Introduction
As SFC continues to expand beyond chiral pharmaceutical applications, reassessing the current columns, parameters and compound classes is required for further development and improvement. In this presentation we focus on alkanes, aromatics, paraffins and other compounds as they apply to the petroleum industry. The first step is the evaluation of the numerous silica columns including HILIC available with the comparison of different particle sizes, column lengths and pore sizes to determine the optimal column. Within this column evaluation, silver impregnated silica columns will be tested and optimized for the retention of alkene (olefins). The second step is to use the best column(s) and study the retention time trends of compounds from the different compound classes by varying the column temperature and back pressure.
After determination of the best silica and silver columns and optimization of column temperature and back pressure, the complete separation is applied to standards and samples for ASTM 5186 and ASTM D6550. In addition, this work will show SFC as an alternative to GC for the separation and identification of hydrocarbons yielding higher resolution and shorter analysis times.
Experimental
Hexadecane, cyclohexane, toluene and naphthalene were obtained from SigmaAldrich. ASTM standards were purchased Spectrum Quality Standards. The JASCO SFC consisting of a CO2 pump, autosampler, column oven, FID detector and back pressure regulator was used for the analysis.
Keywords
SFC 2018, SFC, SFC-FID
Results
Figure 1A shows the comparison of the 6 silica columns from various manufacturers at 120bar back pressure. The JASCO column is the only one that shows any separation of hexadecane and cyclohexane and clearly shows the most resolution between those peaks and the toluene and naphthalene peaks. Figure 1B shows this same comparison at 200bar showing better separation and resolution of hexadecane and cyclohexane and between
those peaks and toluene and naphthalene. Like the JASCO column, the Princeton Chromatography column shows some resolution between hexadecane and cyclohexane.

As the Princeton 60A and the JASCO columns were the only ones to show separation of hexadecane and cyclohexane, those were further evaluated at a few other pressures. As the resolution of those peaks increased when running at 200bar compared to 120bar, 150bar and 250bar were run to confirm the trend and as that higher pressure led to the best separation.

As illustrated in figure 2A, an increase in pressure led to better resolution and 250bar was proven to be the best back pressure on both columns, with the JASCO column proving to be the best. As the pore size of the JASCO column is 30A, a Princeton 30A was compared as well as a Phenomenex Kinetex HILIC column using a simplified 3 component mix. As shown in figure 2B the difference between the Princeton 30A and Princeton 60A is significant as the separation of hexadecane and cyclohexane is drastically improved. The Kinetex® column did not show much retention of any of the peaks. As the pore size was 100A that was likely a factor in the insufficient resolution of hexadecane and cyclohexane, but also the toluene retention time was less than the Halo Penta HILIC.

Figure 3A and figure 3B show that the retention time increases as the back pressure is increased for both hexadecane and cyclohexane. However the magnitude of this increase is different for each. Hexadecane at 100bar elutes at 1.54 minutes and has poor peak shape, while cyclohexane elutes earlier at 1.46 minutes. The peak shape sharpens as the back pressure is increased for hexadecane, but notice the retention time only increases to 1.64 minutes at 300bar compared to later cyclohexane elution at 1.70 minutes. Effectively if these two compounds were in the same mixture their elution order would reverse showing the importance of the back pressure.

Figure 4A, 4B and 4C show the retention behavior of toluene at various temperatures and various temperatures. The retention time does not vary between 35°C and 45°C with the exception at 100bar yielding a slightly longer retention. Although the retention times do not vary, the peak shape is certainly sharper at 35°C. At 55°C the peak shape mimics that broader peak shape seen at 45°C, but the retention time decreases slightly, with the exception of 100bar where the retention time increases. This same data set was repeated using benzene as the sample and the exact same retention behavior was displayed. With benzene, peak broadening was also evident at 45°C and 55°C compared to 35°C, but was much less significant.
ASTM D6650 Results

(Middle). Calibration Curve Correlation Coefficient was 0.997 (Right).
The overlay of 20 chromatograms of the 25% olefin sample is shown in Figure 5A. The overlay of the olefin peak of the various standards, 1.0%, 3.5%, 6.0%, 8.5%, 12.0%, 17.0% and 25%, is shown in figure 5B and the corresponding calibration curve from those standards is also shown in figure 5C. The linearity of the calibration curve had a correlation coefficient (R) of 0.997.
ASTM D5186 Results
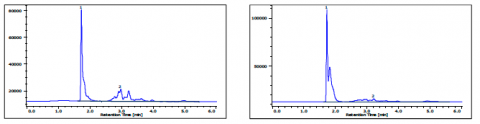
The chromatograms of gasoline and jet fuel under the ASTM D5186 method conditions are shown in figure 6A and figure 6B. The gasoline was determined to have 59.3% non-aromatics and 40.7% aromatics, while the jet fuel had 81.5% non-aromatics and 18.5% aromatics.
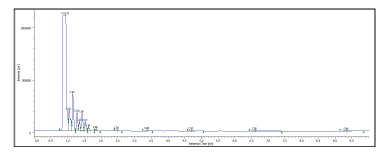
Conclusion
The pore size is a significant factor in the separation efficiency of the column for the separation of alkanes and aromatics. The JASCO 30A packing material provided the best resolution of hexadecane, cyclohexane and toluene with the Princeton 30A a very close 2nd providing equal resolution of hexadecane and cyclohexane, but less resolution of 5.4 compared to 7.7 on the
JASCO silica.
The temperature and pressure studies have not only illustrated how the unique parameter of back pressure plays a significant role in retention time, but also how it can be utilized to optimize a separation when resolution is minimal. Both alkanes and aromatics were more retained at higher pressures, but higher temperatures had the opposite effect on retention and also led to peak broadening. These significant effects from back pressure and temperature provide a user the ability to tune a separation depending on to which class of compounds they are looking to maximize the resolution.
As the results show in the repeatability and reproducibility tables, the SFC-FID exceeds the requirements of the ASTM D6550. Having exceeded the acceptable values, the SFC-FID system can successfully be used for the automated analysis of olefins in gasoline. The ASTM D5186 method was used to successfully analyze commercially available gasoline and jet fuel. The SFC-FID system also offers versatility for high resolution separations of hydrocarbons in a significantly shorter analysis time than provided by GC.
Featured Products:

Evaluation of Temperature and Pressure Parameters for Increased Resolution for the Separation of Alkanes and Aromatics Using SFC
Introduction
As SFC continues to expand beyond chiral pharmaceutical applications, reassessing the current columns, parameters and compound classes is required for further development and improvement. In this presentation we focus on alkanes, aromatics, paraffins and other compounds as they apply to the petroleum industry. The first step is the evaluation of the numerous silica columns including HILIC available with the comparison of different particle sizes, column lengths and pore sizes to determine the optimal column. Within this column evaluation, silver impregnated silica columns will be tested and optimized for the retention of alkene (olefins). The second step is to use the best column(s) and study the retention time trends of compounds from the different compound classes by varying the column temperature and back pressure.
After determination of the best silica and silver columns and optimization of column temperature and back pressure, the complete separation is applied to standards and samples for ASTM 5186 and ASTM D6550. In addition, this work will show SFC as an alternative to GC for the separation and identification of hydrocarbons yielding higher resolution and shorter analysis times.
Experimental
Hexadecane, cyclohexane, toluene and naphthalene were obtained from SigmaAldrich. ASTM standards were purchased Spectrum Quality Standards. The JASCO SFC consisting of a CO2 pump, autosampler, column oven, FID detector and back pressure regulator was used for the analysis.
Results
Figure 1A shows the comparison of the 6 silica columns from various manufacturers at 120bar back pressure. The JASCO column is the only one that shows any separation of hexadecane and cyclohexane and clearly shows the most resolution between those peaks and the toluene and naphthalene peaks. Figure 1B shows this same comparison at 200bar showing better separation and resolution of hexadecane and cyclohexane and between
those peaks and toluene and naphthalene. Like the JASCO column, the Princeton Chromatography column shows some resolution between hexadecane and cyclohexane.

As the Princeton 60A and the JASCO columns were the only ones to show separation of hexadecane and cyclohexane, those were further evaluated at a few other pressures. As the resolution of those peaks increased when running at 200bar compared to 120bar, 150bar and 250bar were run to confirm the trend and as that higher pressure led to the best separation.

As illustrated in figure 2A, an increase in pressure led to better resolution and 250bar was proven to be the best back pressure on both columns, with the JASCO column proving to be the best. As the pore size of the JASCO column is 30A, a Princeton 30A was compared as well as a Phenomenex Kinetex HILIC column using a simplified 3 component mix. As shown in figure 2B the difference between the Princeton 30A and Princeton 60A is significant as the separation of hexadecane and cyclohexane is drastically improved. The Kinetex® column did not show much retention of any of the peaks. As the pore size was 100A that was likely a factor in the insufficient resolution of hexadecane and cyclohexane, but also the toluene retention time was less than the Halo Penta HILIC.

Figure 3A and figure 3B show that the retention time increases as the back pressure is increased for both hexadecane and cyclohexane. However the magnitude of this increase is different for each. Hexadecane at 100bar elutes at 1.54 minutes and has poor peak shape, while cyclohexane elutes earlier at 1.46 minutes. The peak shape sharpens as the back pressure is increased for hexadecane, but notice the retention time only increases to 1.64 minutes at 300bar compared to later cyclohexane elution at 1.70 minutes. Effectively if these two compounds were in the same mixture their elution order would reverse showing the importance of the back pressure.

Figure 4A, 4B and 4C show the retention behavior of toluene at various temperatures and various temperatures. The retention time does not vary between 35°C and 45°C with the exception at 100bar yielding a slightly longer retention. Although the retention times do not vary, the peak shape is certainly sharper at 35°C. At 55°C the peak shape mimics that broader peak shape seen at 45°C, but the retention time decreases slightly, with the exception of 100bar where the retention time increases. This same data set was repeated using benzene as the sample and the exact same retention behavior was displayed. With benzene, peak broadening was also evident at 45°C and 55°C compared to 35°C, but was much less significant.
ASTM D6650 Results

(Middle). Calibration Curve Correlation Coefficient was 0.997 (Right).
The overlay of 20 chromatograms of the 25% olefin sample is shown in Figure 5A. The overlay of the olefin peak of the various standards, 1.0%, 3.5%, 6.0%, 8.5%, 12.0%, 17.0% and 25%, is shown in figure 5B and the corresponding calibration curve from those standards is also shown in figure 5C. The linearity of the calibration curve had a correlation coefficient (R) of 0.997.
ASTM D5186 Results

The chromatograms of gasoline and jet fuel under the ASTM D5186 method conditions are shown in figure 6A and figure 6B. The gasoline was determined to have 59.3% non-aromatics and 40.7% aromatics, while the jet fuel had 81.5% non-aromatics and 18.5% aromatics.

Conclusion
The pore size is a significant factor in the separation efficiency of the column for the separation of alkanes and aromatics. The JASCO 30A packing material provided the best resolution of hexadecane, cyclohexane and toluene with the Princeton 30A a very close 2nd providing equal resolution of hexadecane and cyclohexane, but less resolution of 5.4 compared to 7.7 on the
JASCO silica.
The temperature and pressure studies have not only illustrated how the unique parameter of back pressure plays a significant role in retention time, but also how it can be utilized to optimize a separation when resolution is minimal. Both alkanes and aromatics were more retained at higher pressures, but higher temperatures had the opposite effect on retention and also led to peak broadening. These significant effects from back pressure and temperature provide a user the ability to tune a separation depending on to which class of compounds they are looking to maximize the resolution.
As the results show in the repeatability and reproducibility tables, the SFC-FID exceeds the requirements of the ASTM D6550. Having exceeded the acceptable values, the SFC-FID system can successfully be used for the automated analysis of olefins in gasoline. The ASTM D5186 method was used to successfully analyze commercially available gasoline and jet fuel. The SFC-FID system also offers versatility for high resolution separations of hydrocarbons in a significantly shorter analysis time than provided by GC.
Keywords
SFC 2018, SFC, SFC-FID

 Download This Application
Download This Application

