High-Resolution CPL Spectrum Measurement of a Europium Complex [Eu(facam)3]
January 5, 2024
Introduction
When chiral compounds are excited with unpolarized light, the difference in emission intensities of left- and righthanded circularly polarized light can be measured. This phenomenon is called circularly polarized luminescence (CPL). While circular dichroism provides information about the ground state of chiral molecules, Circular polarized luminescence spectroscopy probes the excited states of chiral molecules.
In recent years, CPL-active molecules have been used for a wide range of technical applications such as security encoding, bioanalytical probes, and liquid crystal display devices.1 Chiral lanthanide complexes are one example of target molecules for CPL measurements. These complexes are known to exhibit sharp emission bands and require a narrow bandwidth during CPL measurements.
JASCO has recently developed a high sensitivity CPL spectrometer. By combining CPL with ECD, more structural information regarding chiral molecules can be obtained. To measure sharp CPL peaks at a high resolution, JASCO’s CPL-300 spectrometer uses two prism monochromators. Both the emission and excitation monochromators are equipped with continuously variable slit drives, which allow for an appropriate wavelength and bandwidth selection.
This application note demonstrates the high-resolution CPL spectrum measurement of europium tris[3-(trifluoromethylhydroxymethylene)-(+)-camphorate] (Eu(facam)3) using a CPL-300. Eu(facam)3 is a common NMR shift reagent and has been used as a standard for CPL measurements.2

Experimental
5.5 mM Eu(facam)3 was prepared in DMSO.
| Measurement conditions | |
|---|---|
| Excitation wavelength | 373 nm |
| Excitation slit width | 4000 µm |
| Emission bandwidth | 3 nm |
| Scan speed | 200 nm/min |
| Response time | 4 sec |
| Data acquisition interval | 0.1 nm |
| Accumulations | 4 |
| Path length | 10 mm |
The absorption spectrum of Eu(facam)3 /DMSO solution was measured using a V-760 spectrometer and a 0.1 mm pathlength cylindrical cell and holder. The fluorescence spectra were obtained using a FP-8300 spectrofluorimeter and a 10×10 mm pathlength rectangular cell.
Keywords
180-CD-0028, Circularly Polarized Luminescence, CPL, lanthanides, luminescent material
Results
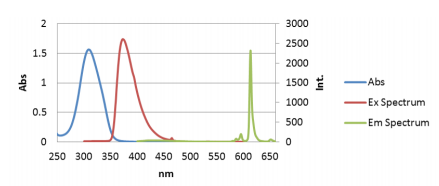
In order to find the excitation maximum needed to obtain the CPL and fluorescence emission spectrum of Eu(facam)3/ DMSO, the absorption spectrum was first obtained. Figure 1 depicts a maximum absorption peak (blue) at 309 nm. However, in order to optimize the excitation wavelength used in the CPL and fluorescence measurements, an excitation spectrum was subsequentially measured at the expected emission maximum peak’s wavelength at 613 nm. The excitation spectrum shows an apparent maximum at 373 nm (Figure 1, red). The excitation wavelength was set to 373 nm and the CPL and fluorescence measurements of Eu(facam)3/DMSO were measured and are shown in Figure 2.
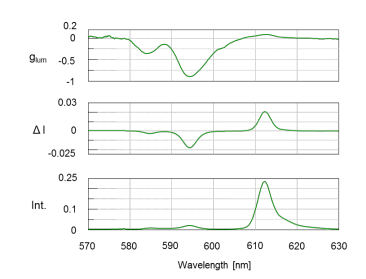
All three spectra in Figure 2 show the 5D0 –> 7 F1 magnetic-dipole transition band at 595 nm. The additional emission band at 611 nm is due to the 5D0 –> 7 F2 electronic-dipole transition.3 The CPL spectrum shows strong signals which confirms the presence of the chiral facam ligands. The degree of CPL can be described by glum, the luminescent dissymmetry factor. This value quantifies the asymmetric environment surrounding the complexes’ metal ions. The larger the dissymmetry factor, the more polarized the emitted light will be. A glum of ±2 indicates the complete polarization of light while 0 correlates to unpolarized emitted light. Figure 2 (top) shows a glum value ca. -0.8 for the 595 nm transition band, indicating a chiral species is present.
Conclusion
The JASCO CPL-300 spectrometer can perform CPL measurements that produce high resolution spectra. The CPL, fluorescence, and glum spectra are all consistent with the literature.2,4,5
References
1. F Zinna and L Di Bari, Chirality, 2015, 27:1.
2. HG Brittain and FS Richardson, JACS, 1976, 98: 5858
3. JP Riehl, Chemical Reviews, 1986, 86: 1.
4. CK Luk and FS Richardson, JACS, 1975, 97: 6666.
5. T Harada, Y Nakano, M Fujiki, M Naito, T Kawai, and Y Hasegawa, Inorganic Chemistry, 2009, 48: 11242.
Featured Products:

High-Resolution CPL Spectrum Measurement of a Europium Complex [Eu(facam)3]
Introduction
When chiral compounds are excited with unpolarized light, the difference in emission intensities of left- and righthanded circularly polarized light can be measured. This phenomenon is called circularly polarized luminescence (CPL). While circular dichroism provides information about the ground state of chiral molecules, Circular polarized luminescence spectroscopy probes the excited states of chiral molecules.
In recent years, CPL-active molecules have been used for a wide range of technical applications such as security encoding, bioanalytical probes, and liquid crystal display devices.1 Chiral lanthanide complexes are one example of target molecules for CPL measurements. These complexes are known to exhibit sharp emission bands and require a narrow bandwidth during CPL measurements.
JASCO has recently developed a high sensitivity CPL spectrometer. By combining CPL with ECD, more structural information regarding chiral molecules can be obtained. To measure sharp CPL peaks at a high resolution, JASCO’s CPL-300 spectrometer uses two prism monochromators. Both the emission and excitation monochromators are equipped with continuously variable slit drives, which allow for an appropriate wavelength and bandwidth selection.
This application note demonstrates the high-resolution CPL spectrum measurement of europium tris[3-(trifluoromethylhydroxymethylene)-(+)-camphorate] (Eu(facam)3) using a CPL-300. Eu(facam)3 is a common NMR shift reagent and has been used as a standard for CPL measurements.2

Experimental
5.5 mM Eu(facam)3 was prepared in DMSO.
| Measurement conditions | |
|---|---|
| Excitation wavelength | 373 nm |
| Excitation slit width | 4000 µm |
| Emission bandwidth | 3 nm |
| Scan speed | 200 nm/min |
| Response time | 4 sec |
| Data acquisition interval | 0.1 nm |
| Accumulations | 4 |
| Path length | 10 mm |
The absorption spectrum of Eu(facam)3 /DMSO solution was measured using a V-760 spectrometer and a 0.1 mm pathlength cylindrical cell and holder. The fluorescence spectra were obtained using a FP-8300 spectrofluorimeter and a 10×10 mm pathlength rectangular cell.
Results
In order to find the excitation maximum needed to obtain the CPL and fluorescence emission spectrum of Eu(facam)3/ DMSO, the absorption spectrum was first obtained. Figure 1 depicts a maximum absorption peak (blue) at 309 nm. However, in order to optimize the excitation wavelength used in the CPL and fluorescence measurements, an excitation spectrum was subsequentially measured at the expected emission maximum peak’s wavelength at 613 nm. The excitation spectrum shows an apparent maximum at 373 nm (Figure 1, red). The excitation wavelength was set to 373 nm and the CPL and fluorescence measurements of Eu(facam)3/DMSO were measured and are shown in Figure 2.


All three spectra in Figure 2 show the 5D0 –> 7 F1 magnetic-dipole transition band at 595 nm. The additional emission band at 611 nm is due to the 5D0 –> 7 F2 electronic-dipole transition.3 The CPL spectrum shows strong signals which confirms the presence of the chiral facam ligands. The degree of CPL can be described by glum, the luminescent dissymmetry factor. This value quantifies the asymmetric environment surrounding the complexes’ metal ions. The larger the dissymmetry factor, the more polarized the emitted light will be. A glum of ±2 indicates the complete polarization of light while 0 correlates to unpolarized emitted light. Figure 2 (top) shows a glum value ca. -0.8 for the 595 nm transition band, indicating a chiral species is present.
Conclusion
The JASCO CPL-300 spectrometer can perform CPL measurements that produce high resolution spectra. The CPL, fluorescence, and glum spectra are all consistent with the literature.2,4,5
Keywords
180-CD-0028, Circularly Polarized Luminescence, CPL, lanthanides, luminescent material
References
1. F Zinna and L Di Bari, Chirality, 2015, 27:1.
2. HG Brittain and FS Richardson, JACS, 1976, 98: 5858
3. JP Riehl, Chemical Reviews, 1986, 86: 1.
4. CK Luk and FS Richardson, JACS, 1975, 97: 6666.
5. T Harada, Y Nakano, M Fujiki, M Naito, T Kawai, and Y Hasegawa, Inorganic Chemistry, 2009, 48: 11242.

 Download This Application
Download This Application