High Sensitivity Detection of Polycyclic Aromatic Hydrocarbons using Fluorescence Detection in SFC
October 9, 2024
Introduction
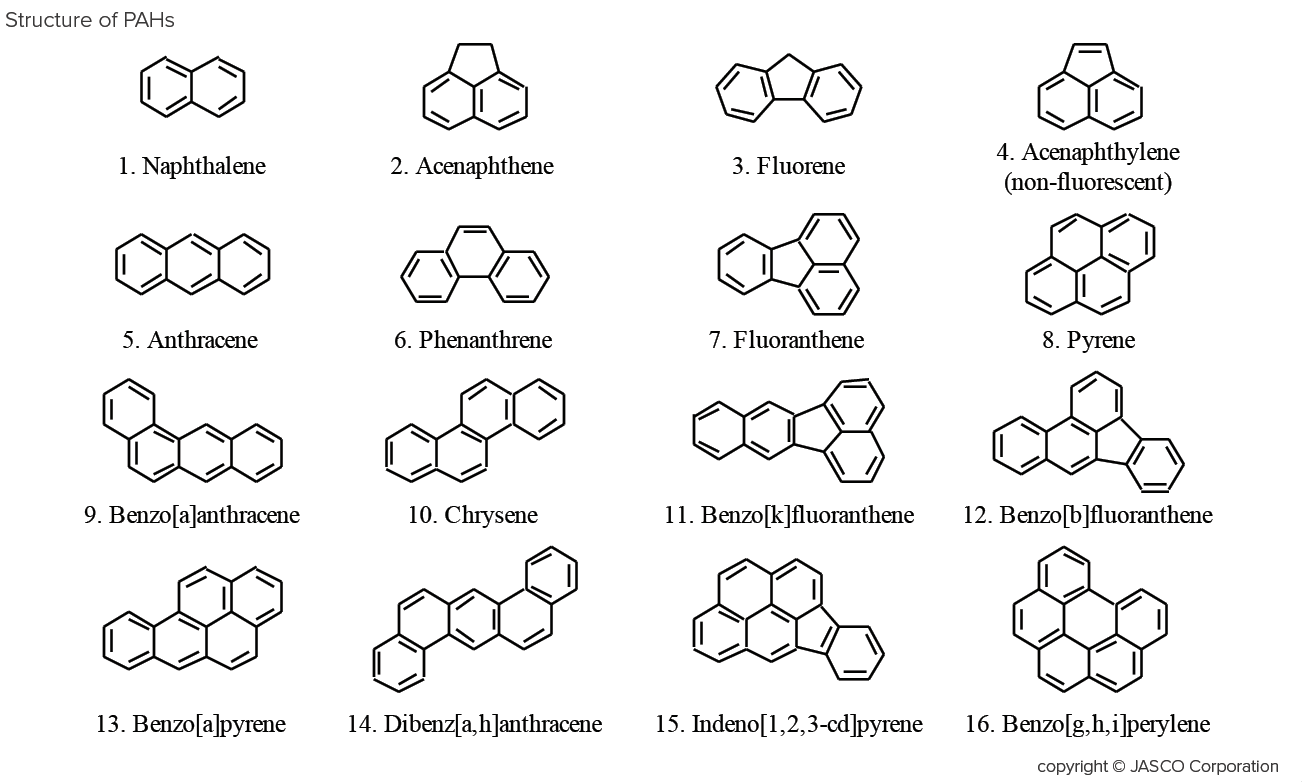
Polycyclic Aromatic Hydrocarbons (PAHs) are produced by the incomplete combustion of organic compounds from various sources including diesel exhaust, coal dust, and cigarette smoke; many are strongly carcinogenic. PAHs are subject to regulation by various organizations including the EPA (United States Environmental Protection Agency). These regulations strictly control the impact of PAHs on environmental pollution and their effects on human health.
PAHs exhibit strong natural fluorescence due to the aromatic structure with many conjugated double bonds. This makes the high sensitivity of fluorescence detection necessary for the low levels often found in the environment.
In this application note, 16 PAHs were separated by supercritical fluid chromatography (SFC) and detected using a FP-4020 fluorescence detector (FL detector) with a high pressure flow cell (previously introduced in LC application data No.032006U). Data was collected using both UV-Vis and fluorescence detectors for comparative purposes.
Experimental
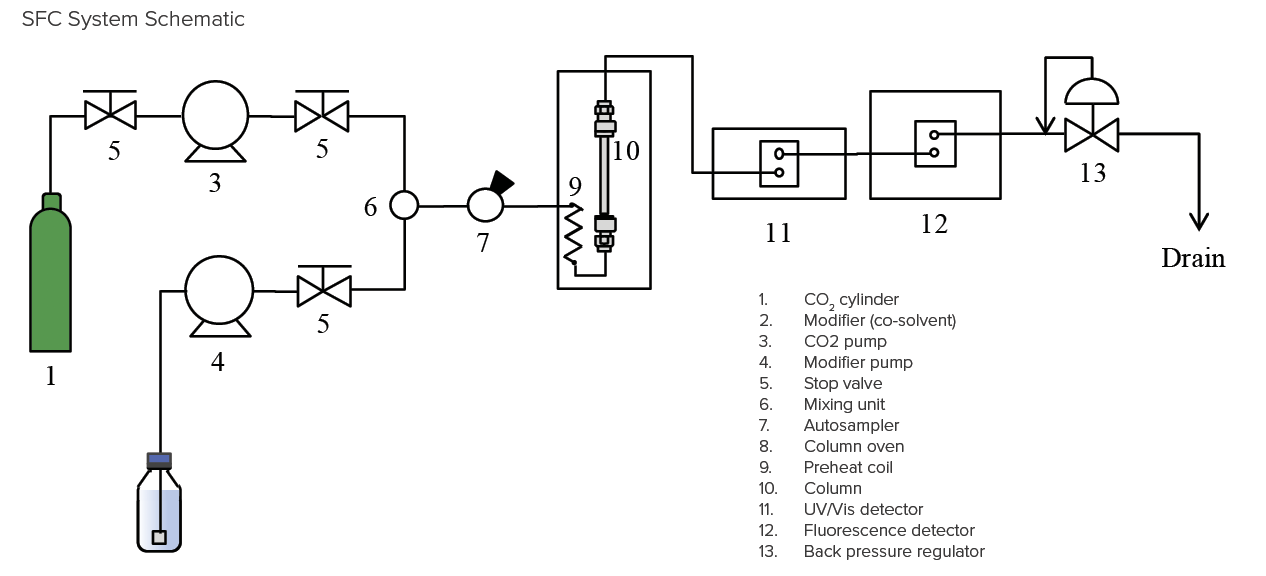
| Equipment | |
| CO2 Pump | PU-4380 |
| Modifier Pump | PU-4180 |
| Modifier Pump Option | SV Unit MX Unit |
| Autosampler | AS-4350 |
| Column Oven | CO-4060 |
| UV/Vis Detector | UV-4070 |
| UV/Vis Detector Flow Cell | Analytical High Pressure |
| FL Detector | FP-4020 |
| FL Detector Flow Cell | Analytical High Pressure |
| Back Pressure Regulator | BP-4340 |
| Conditions | |
| Column | 2-Ethylpyridine (Princeton Chromatography) (4.6 mm I.D. x 250 mm L, 5 µm) |
| Eluent A | CO2 |
| Eluent B | Acetonitrile |
| Gradient | (A/B), 0 min (95/5) → 1.5 min (95/5) → 12 min (80/20) → 14 min (80/20) → 15 min (95/5) → 20 min (95/5) |
| Flow Rate | 3.0 mL/min |
| Column Temp. | 40 ̊C |
| Wavelength | Refer to Figure 1 |
| Back Pressure | 15 MPa |
| Injection Volume | 5 µL |
| Standard Sample | Mixture of 16 PAHs standards in acetonitrile |
Keywords
230009U, Polycyclic Aromatic Hydrocarbons, PAHs, Ethylpyridine column, Supercritical Fluid Chromatography, SFC, Fluorescence detector, carcinogenicity, UV-Vis, absorbance detector
Results
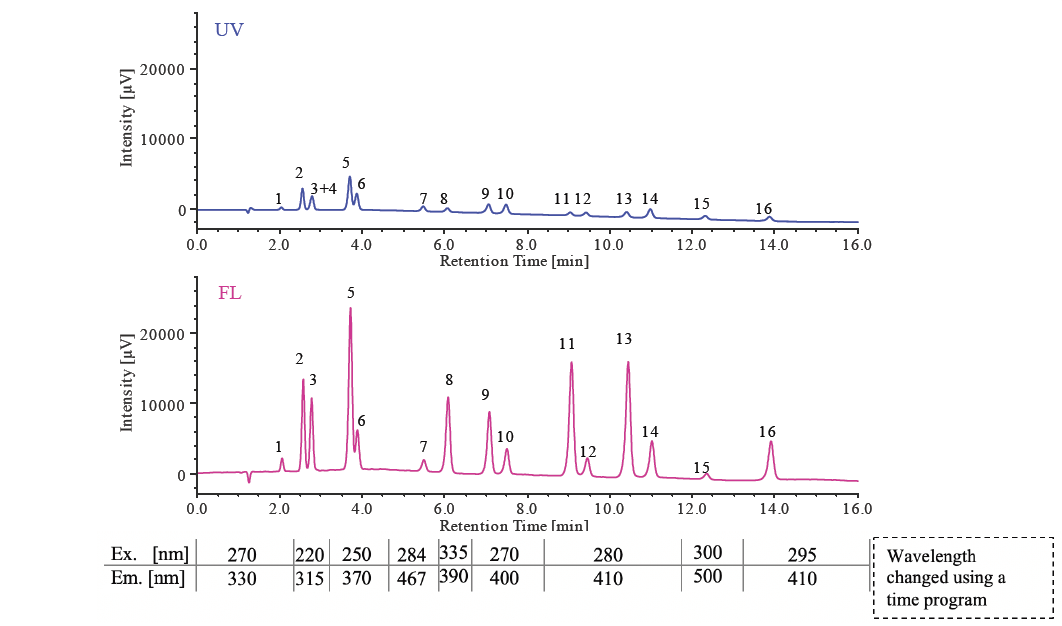
Figure 1 shows the separation of a 16-component PAHs standard mixture with UV-visible detection (500 pg/µL each) and fluorescence detection (5 pg/µL each). Since Acenaphthylene does not fluoresce, it was not detected in the fluorescence chromatogram and was co-eluted with Fluorene (peak No. 3) overlapping in the UV chromatogram. Table 1 shows the detection limits of each component.
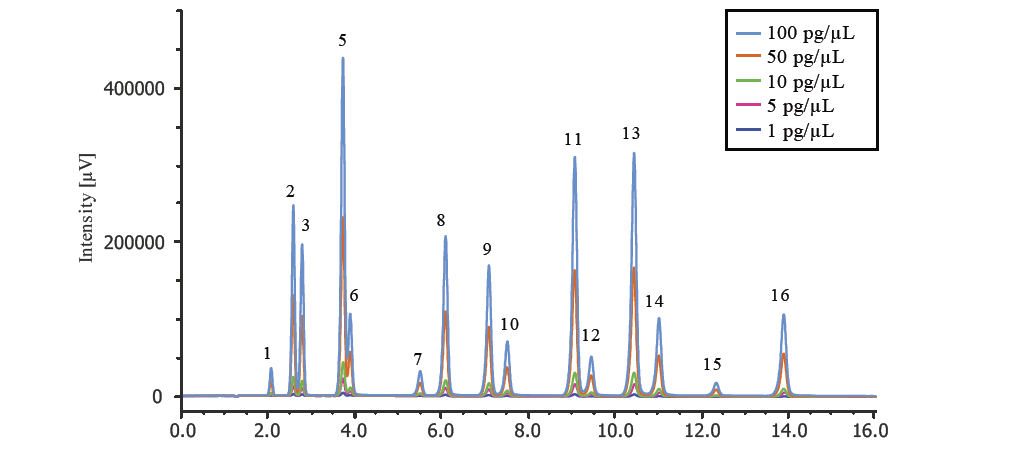
| Peak No. | Compound | Detection Limit [pg] | Sensitivity Ratio*3 | Correlation coefficient (FL)*4 | |
| UV*1 | FL*2 | ||||
| 1 | Naphthalene | 120 | 2.04 | 59 | 1.0000 |
| 2 | Acenaphthene | 13.6 | 0.293 | 46 | 0.9998 |
| 3 | Fluorene*5 | 21.3 | 0.369 | 58 | 0.9999 |
| 5 | Anthracene | 8.67 | 0.166 | 52 | 0.9998 |
| 6 | Phenanthrene | 17.8 | 0.676 | 26 | 1.0000 |
| 7 | Fluoranthene | 58.8 | 2.34 | 25 | 0.9999 |
| 8 | Pyrene | 75.2 | 0.357 | 211 | 0.9998 |
| 9 | Benzo[a]anthrancene | 32.5 | 0.432 | 75 | 0.9999 |
| 10 | Chrysene | 31.5 | 1.06 | 30 | 1.0000 |
| 11 | Benzo[k]fluoranthene | 93.0 | 0.236 | 393 | 0.9997 |
| 12 | Benzo[b]fluoranthene | 81.9 | 1.46 | 56 | 0.9999 |
| 13 | Benzo[a]pyrene | 53.6 | 0.233 | 230 | 0.9998 |
| 14 | Dibenz[a,h]anthracene | 32.6 | 0.737 | 44 | 1.0000 |
| 15 | Indeno[1,2,3-cd]pyrene | 79.2 | 4.61 | 17 | 0.9998 |
| 16 | Benzo[g,h,i]perylene | 65.5 | 0.697 | 94 | 0.9998 |
| *1 Calculated from the measurement of 500 pg/mL standard sample (S/N=3) *2 Calculated from the measurement of 5 pg/mL standard sample (S/N=3) *3 Lower limit of detection of UV detector/Lower limit of detection of FL detector *4 Calibration curve was made using standards at 1, 5, 10, 50, 100 pg/mL *5 The value of the Fluorene peak using UV detection includes Acenaphthylene which is co-eluted. |
|||||
Featured Products:

High Sensitivity Detection of Polycyclic Aromatic Hydrocarbons using Fluorescence Detection in SFC
Introduction
Polycyclic Aromatic Hydrocarbons (PAHs) are produced by the incomplete combustion of organic compounds from various sources including diesel exhaust, coal dust, and cigarette smoke; many are strongly carcinogenic. PAHs are subject to regulation by various organizations including the EPA (United States Environmental Protection Agency). These regulations strictly control the impact of PAHs on environmental pollution and their effects on human health.
PAHs exhibit strong natural fluorescence due to the aromatic structure with many conjugated double bonds. This makes the high sensitivity of fluorescence detection necessary for the low levels often found in the environment.
In this application note, 16 PAHs were separated by supercritical fluid chromatography (SFC) and detected using a FP-4020 fluorescence detector (FL detector) with a high pressure flow cell (previously introduced in LC application data No.032006U). Data was collected using both UV-Vis and fluorescence detectors for comparative purposes.
Experimental
| Equipment | |
| CO2 Pump | PU-4380 |
| Modifier Pump | PU-4180 |
| Modifier Pump Option | SV Unit MX Unit |
| Autosampler | AS-4350 |
| Column Oven | CO-4060 |
| UV/Vis Detector | UV-4070 |
| UV/Vis Detector Flow Cell | Analytical High Pressure |
| FL Detector | FP-4020 |
| FL Detector Flow Cell | Analytical High Pressure |
| Back Pressure Regulator | BP-4340 |
| Conditions | |
| Column | 2-Ethylpyridine (Princeton Chromatography) (4.6 mm I.D. x 250 mm L, 5 µm) |
| Eluent A | CO2 |
| Eluent B | Acetonitrile |
| Gradient | (A/B), 0 min (95/5) → 1.5 min (95/5) → 12 min (80/20) → 14 min (80/20) → 15 min (95/5) → 20 min (95/5) |
| Flow Rate | 3.0 mL/min |
| Column Temp. | 40 ̊C |
| Wavelength | Refer to Figure 1 |
| Back Pressure | 15 MPa |
| Injection Volume | 5 µL |
| Standard Sample | Mixture of 16 PAHs standards in acetonitrile |


Results
Figure 1 shows the separation of a 16-component PAHs standard mixture with UV-visible detection (500 pg/µL each) and fluorescence detection (5 pg/µL each). Since Acenaphthylene does not fluoresce, it was not detected in the fluorescence chromatogram and was co-eluted with Fluorene (peak No. 3) overlapping in the UV chromatogram. Table 1 shows the detection limits of each component.

| Peak No. | Compound | Detection Limit [pg] | Sensitivity Ratio*3 | Correlation coefficient (FL)*4 | |
| UV*1 | FL*2 | ||||
| 1 | Naphthalene | 120 | 2.04 | 59 | 1.0000 |
| 2 | Acenaphthene | 13.6 | 0.293 | 46 | 0.9998 |
| 3 | Fluorene*5 | 21.3 | 0.369 | 58 | 0.9999 |
| 5 | Anthracene | 8.67 | 0.166 | 52 | 0.9998 |
| 6 | Phenanthrene | 17.8 | 0.676 | 26 | 1.0000 |
| 7 | Fluoranthene | 58.8 | 2.34 | 25 | 0.9999 |
| 8 | Pyrene | 75.2 | 0.357 | 211 | 0.9998 |
| 9 | Benzo[a]anthrancene | 32.5 | 0.432 | 75 | 0.9999 |
| 10 | Chrysene | 31.5 | 1.06 | 30 | 1.0000 |
| 11 | Benzo[k]fluoranthene | 93.0 | 0.236 | 393 | 0.9997 |
| 12 | Benzo[b]fluoranthene | 81.9 | 1.46 | 56 | 0.9999 |
| 13 | Benzo[a]pyrene | 53.6 | 0.233 | 230 | 0.9998 |
| 14 | Dibenz[a,h]anthracene | 32.6 | 0.737 | 44 | 1.0000 |
| 15 | Indeno[1,2,3-cd]pyrene | 79.2 | 4.61 | 17 | 0.9998 |
| 16 | Benzo[g,h,i]perylene | 65.5 | 0.697 | 94 | 0.9998 |
| *1 Calculated from the measurement of 500 pg/mL standard sample (S/N=3) *2 Calculated from the measurement of 5 pg/mL standard sample (S/N=3) *3 Lower limit of detection of UV detector/Lower limit of detection of FL detector *4 Calibration curve was made using standards at 1, 5, 10, 50, 100 pg/mL *5 The value of the Fluorene peak using UV detection includes Acenaphthylene which is co-eluted. |
|||||

Keywords
230009U, Polycyclic Aromatic Hydrocarbons, PAHs, Ethylpyridine column, Supercritical Fluid Chromatography, SFC, Fluorescence detector, carcinogenicity, UV-Vis, absorbance detector

 Download This Application
Download This Application
