Magnetic Circular Dichroism using a PMCD-586 Permanent Magnet
January 5, 2024
Introduction
Permanent Magnetic Circular Dichroism
Magnetic circular dichroism passes linearly polarized light through a material in a magnetic field parallel to the direction of the magnetic field, and the polarization plane is rotated. The use of a magnetic field to induce optical activity in molecules is also known as the Faraday effect.
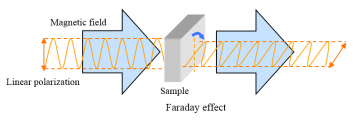
Magnetic Optical Rotation Dispersion (MORD) and Magnetic Circular Dichroism (MCD) can be used to observe magnetic field-induced effects on optically active molecules. MCD is widely used today to probe the local environment of protein molecules. Chromophores with large magnetic moments arising from either rotational symmetries (aromatics, porphyrins), unpaired spins (metal complexes), or both (hemes) are sensitive to electronic perturbations and therefore provide information regarding the molecule’s electronic state. The MCD signal intensity is proportional to the magnetic field strength, which can be applied by using a permanent magnet.
Previously, in order to generate a strong magnetic field (greater than 1 Tesla) a large electromagnet was required. However, electromagnets are not easily located inside the sample compartment of a CD spectrometer due to its excessive weight (typically greater than 60 kg). The PMCD-586 permanent magnet has a magnetic field strength of 1.6 Tesla and easily fits inside the sample compartment. The direction of the magnetic field can be changed by simply reversing the direction of the magnet.
This application note provides examples of the J-1500 CD spectrometer and PMCD-586 permanent magnet to obtain MCD measurements.
Experimental
Measurement conditions
| Neodymium glass | Cytochrome C | Cobalt chloride (II) 6 Hydrate |
|
|---|---|---|---|
| Data Acquisition Interval | 0.5 seconds | 2 seconds | 2 seconds |
| Spectral Bandwidth | 1 nm | 1 nm | 1 nm |
| Scan Speed | 50 nm/min/td> | 20 nm/min | 100 nm/min |
| Path Length | 0.2 cm | 0.5 cm |
Keywords
180-CD-0008, J-1500, circular dichroism, magnetic circular dichroism, Faraday effect, UV-Vis, NIR, biochemistry, chemical
Results
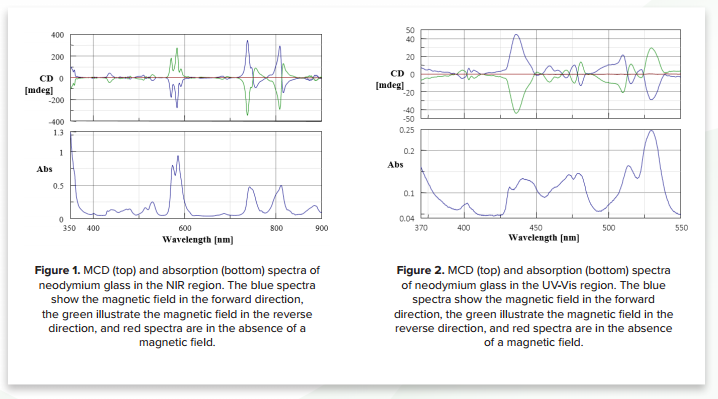
Neodymium glass is optically inactive. However, when subjected to a magnetic field an MCD spectrum from the UVVisible to NIR region can be observed. The spectra in Figure 1 show sharp MCD signals caused by strong optical absorption. Figure 2 shows that small MCD signals caused by weak optical absorptions can be enhanced by the strong 1.6 T magnetic field. MCD spectra illustrate sharp peaks that cannot be separated in the absorption spectra.
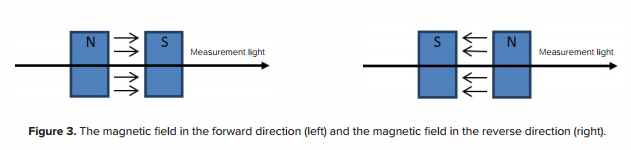
If the direction of the magnetic field is the same as that of the incoming light, the magnetic field can be defined as being in the forward direction. If the direction of the magnetic field is opposite to that of the incoming light, it is defined as the magnetic field in the reverse direction. This is depicted in Figure 3. By changing the direction of the magnetic field, the MCD spectra can also be reversed.
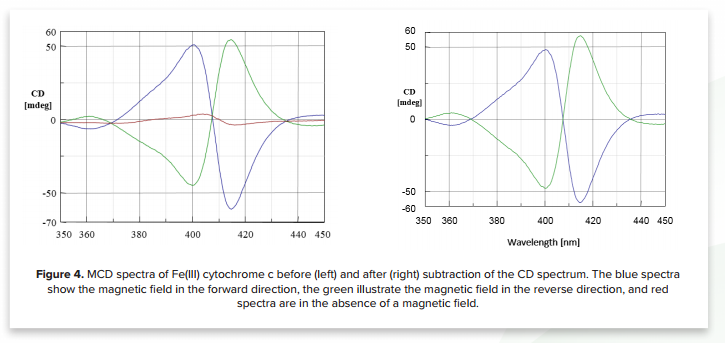
MCD of Fe (III) cytochrome c
MCD is widely used for the structural analysis of hemoprotein samples and several studies of myoglobin¹, hemoglobin², cytochrome b₅3-4, cytochrome c3,5-6, cytochrome P-4507 , and horseradish peroxidase8 are reported. The MCD spectra of the Soret band of Fe(III) cytochrome in solution are shown in Figure 4. If the CD spectrum obtained in the absence of a magnetic field is subtracted from the MCD spectra, symmetric MCD spectra can be observed.
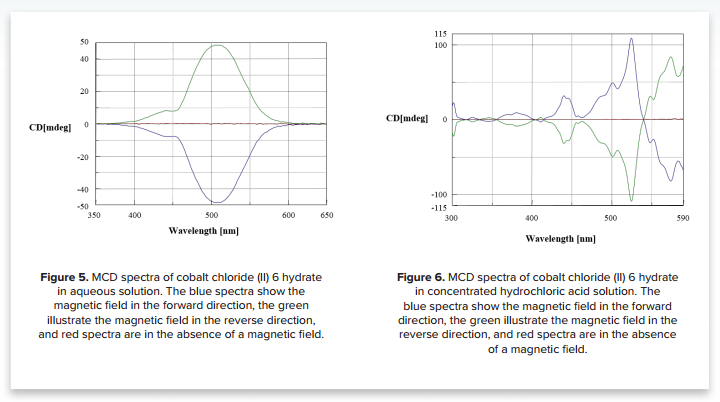
MCD of cobalt chloride (II) 6 hydrate
An aqueous solution of cobalt chloride (II) 6 hydrate exists as a hexacoordinated structure where the cobalt ion is coordinated with six water molecules. Conversely, cobalt chloride 6 hydrate in a concentrated HCl solution exists as a tetrahedron complex in which the cobalt ion is coordinated to four chloride ions. The MCD spectra illustrate drastic changes based on the change in the electronic state of the cobalt ion and are shown in Figures 5 and 6.
Conclusion
This application note illustrates that the J-1500 CD spectrometer and the PMCD-586 permanent magnet can be used to obtain magnetic circular dichroism spectra for a variety of samples.
Featured Products:
-

Highest performance with a wide range of accessories for maximum flexibility to meet complex research demands.
J-1500
-
Magnetic CD

Magnetic Circular Dichroism using a PMCD-586 Permanent Magnet
Introduction
Permanent Magnetic Circular Dichroism
Magnetic circular dichroism passes linearly polarized light through a material in a magnetic field parallel to the direction of the magnetic field, and the polarization plane is rotated. The use of a magnetic field to induce optical activity in molecules is also known as the Faraday effect.

Magnetic Optical Rotation Dispersion (MORD) and Magnetic Circular Dichroism (MCD) can be used to observe magnetic field-induced effects on optically active molecules. MCD is widely used today to probe the local environment of protein molecules. Chromophores with large magnetic moments arising from either rotational symmetries (aromatics, porphyrins), unpaired spins (metal complexes), or both (hemes) are sensitive to electronic perturbations and therefore provide information regarding the molecule’s electronic state. The MCD signal intensity is proportional to the magnetic field strength, which can be applied by using a permanent magnet.
Previously, in order to generate a strong magnetic field (greater than 1 Tesla) a large electromagnet was required. However, electromagnets are not easily located inside the sample compartment of a CD spectrometer due to its excessive weight (typically greater than 60 kg). The PMCD-586 permanent magnet has a magnetic field strength of 1.6 Tesla and easily fits inside the sample compartment. The direction of the magnetic field can be changed by simply reversing the direction of the magnet.
This application note provides examples of the J-1500 CD spectrometer and PMCD-586 permanent magnet to obtain MCD measurements.
Experimental
Measurement conditions
| Neodymium glass | Cytochrome C | Cobalt chloride (II) 6 Hydrate |
|
|---|---|---|---|
| Data Acquisition Interval | 0.5 seconds | 2 seconds | 2 seconds |
| Spectral Bandwidth | 1 nm | 1 nm | 1 nm |
| Scan Speed | 50 nm/min/td> | 20 nm/min | 100 nm/min |
| Path Length | 0.2 cm | 0.5 cm |
Keywords
180-CD-0008, J-1500, circular dichroism, magnetic circular dichroism, Faraday effect, UV-Vis, NIR, biochemistry, chemical
Results
Neodymium glass is optically inactive. However, when subjected to a magnetic field an MCD spectrum from the UVVisible to NIR region can be observed. The spectra in Figure 1 show sharp MCD signals caused by strong optical absorption. Figure 2 shows that small MCD signals caused by weak optical absorptions can be enhanced by the strong 1.6 T magnetic field. MCD spectra illustrate sharp peaks that cannot be separated in the absorption spectra.

If the direction of the magnetic field is the same as that of the incoming light, the magnetic field can be defined as being in the forward direction. If the direction of the magnetic field is opposite to that of the incoming light, it is defined as the magnetic field in the reverse direction. This is depicted in Figure 3. By changing the direction of the magnetic field, the MCD spectra can also be reversed.

MCD of Fe (III) cytochrome c
MCD is widely used for the structural analysis of hemoprotein samples and several studies of myoglobin¹, hemoglobin², cytochrome b₅3-4, cytochrome c3,5-6, cytochrome P-4507 , and horseradish peroxidase8 are reported. The MCD spectra of the Soret band of Fe(III) cytochrome in solution are shown in Figure 4. If the CD spectrum obtained in the absence of a magnetic field is subtracted from the MCD spectra, symmetric MCD spectra can be observed.

MCD of cobalt chloride (II) 6 hydrate
An aqueous solution of cobalt chloride (II) 6 hydrate exists as a hexacoordinated structure where the cobalt ion is coordinated with six water molecules. Conversely, cobalt chloride 6 hydrate in a concentrated HCl solution exists as a tetrahedron complex in which the cobalt ion is coordinated to four chloride ions. The MCD spectra illustrate drastic changes based on the change in the electronic state of the cobalt ion and are shown in Figures 5 and 6.

Conclusion
This application note illustrates that the J-1500 CD spectrometer and the PMCD-586 permanent magnet can be used to obtain magnetic circular dichroism spectra for a variety of samples.

 Download This Application
Download This Application
