Mass-Directed Preparative SFC – Recovery and Purity Determination
August 19, 2022
Introduction
Supercritical fluid chromatography (SFC) is a rapidly growing technique. Initally used almost exclusively only for thermally labile and chiral compounds, it is now accepted as a versatile analytical and purification technique applied across a broad range of chiral and achiral compounds. It offers a higher-throughput than regular HPLC and Prep-LC and offers the ‘green’ advantages of much lower solvent consumption, consumable and disposal costs. SFC is compatible with a wide range of detection methods including UV, electrospray (ESI) and atmospheric pressure chemical ionization (APCI) mass spectrometry.

Methods
The initial tests explored a wide range of flow rates, both isocratic and gradient, to test performance and reliability of the passive splitter, coincident timing of MS and UV signal and the clearance of the source with highly concentrated samples. The system was further evaluated by testing the recovery and purity of compounds in a three-component mixture.
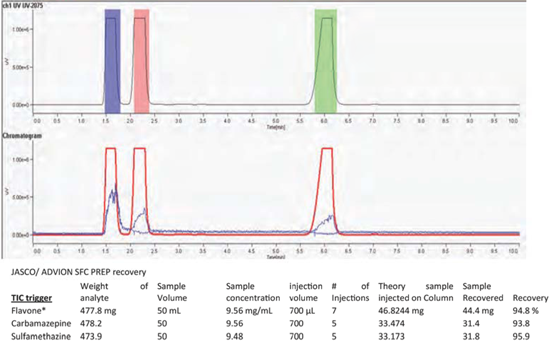
Test #1: Three component standard mixture, 700 μL injections, gradient elution, mass-directed collection using TIC of each peak per injection starting with Peak A, then B & C (Flavone, Carbamazepine and Sulfamethazine). Repeat for a total of 5 injections. Measure the purity and recovery of the three collected fractions.
Test #2: Three component standard mixture , 700 μL injections, gradient elution, UV-directed collection using UV of each peaks per injection starting with Peak A, then B & C (Flavone, Carbamazepine, and Sulfamethazine). Repeat for a total of 5 injections. Measure the purity and recovery of the three collected fractions.
Experimental
| Mass Spectrometer: | expression CMS |
| SFC: | Prep-2088 SFC System |
| Total Flow: | 70 ml/min |
| BPR: | 120 bar |
| Column: | Silica 2-Ethylpyridine OBD Prep Column, 100A, 5 μm, 19 mm x 250 mm |
| Co-Solvent: | Methanol |
| Gradient: | 5-25% in 7 min. |
| Splitter Configuration: | 100cm (2 x 50 cm) PEEKSil 0.025 mm ID x 1/16' OD coupled with ZDV union |
| Makeup Pump Flow: | 8 mL/min Me0H (constant flow for 30% modifier and below) |
| Interface: | ChromNav setup to acquire two analog outputs from expression CMS - total ion chromatogram (TIC) and extracted ion chromatogram (XIC)mg/mL |
| Sample: | Flavone 9.56 mg/mL Carbamazepine 9.56 mg/mL Sulfamethazine 9.48 mg/mL |
Results
TIC triggered fraction collection (5 injections)
UV triggered Fraction collection (5 injections)

Conclusion
- The CMS can be simply interfaced to both analytical scale and prep SFC using a simple passive split before the UV detector.
- Timing delay between UV and MS signals was determined to be approx. 3.6 seconds .
- Fractions collected for each analyte were tested and showed that each was 99.9% pure or greater.
- The difference between the recoveries using UV-triggering verses fractions triggered by MS-TIC was less than 2% for the flavone and sulfamethazine peak and 6% for the carbamazepine.
Featured Products:

Mass-Directed Preparative SFC – Recovery and Purity Determination
Introduction
Supercritical fluid chromatography (SFC) is a rapidly growing technique. Initally used almost exclusively only for thermally labile and chiral compounds, it is now accepted as a versatile analytical and purification technique applied across a broad range of chiral and achiral compounds. It offers a higher-throughput than regular HPLC and Prep-LC and offers the ‘green’ advantages of much lower solvent consumption, consumable and disposal costs. SFC is compatible with a wide range of detection methods including UV, electrospray (ESI) and atmospheric pressure chemical ionization (APCI) mass spectrometry.

Methods
The initial tests explored a wide range of flow rates, both isocratic and gradient, to test performance and reliability of the passive splitter, coincident timing of MS and UV signal and the clearance of the source with highly concentrated samples. The system was further evaluated by testing the recovery and purity of compounds in a three-component mixture.
Test #1: Three component standard mixture, 700 μL injections, gradient elution, mass-directed collection using TIC of each peak per injection starting with Peak A, then B & C (Flavone, Carbamazepine and Sulfamethazine). Repeat for a total of 5 injections. Measure the purity and recovery of the three collected fractions.
Test #2: Three component standard mixture , 700 μL injections, gradient elution, UV-directed collection using UV of each peaks per injection starting with Peak A, then B & C (Flavone, Carbamazepine, and Sulfamethazine). Repeat for a total of 5 injections. Measure the purity and recovery of the three collected fractions.
Experimental
| Mass Spectrometer: | expression CMS |
| SFC: | Prep-2088 SFC System |
| Total Flow: | 70 ml/min |
| BPR: | 120 bar |
| Column: | Silica 2-Ethylpyridine OBD Prep Column, 100A, 5 μm, 19 mm x 250 mm |
| Co-Solvent: | Methanol |
| Gradient: | 5-25% in 7 min. |
| Splitter Configuration: | 100cm (2 x 50 cm) PEEKSil 0.025 mm ID x 1/16' OD coupled with ZDV union |
| Makeup Pump Flow: | 8 mL/min Me0H (constant flow for 30% modifier and below) |
| Interface: | ChromNav setup to acquire two analog outputs from expression CMS - total ion chromatogram (TIC) and extracted ion chromatogram (XIC)mg/mL |
| Sample: | Flavone 9.56 mg/mL Carbamazepine 9.56 mg/mL Sulfamethazine 9.48 mg/mL |
Results
TIC triggered fraction collection (5 injections)

UV triggered Fraction collection (5 injections)

Conclusion
- The CMS can be simply interfaced to both analytical scale and prep SFC using a simple passive split before the UV detector.
- Timing delay between UV and MS signals was determined to be approx. 3.6 seconds .
- Fractions collected for each analyte were tested and showed that each was 99.9% pure or greater.
- The difference between the recoveries using UV-triggering verses fractions triggered by MS-TIC was less than 2% for the flavone and sulfamethazine peak and 6% for the carbamazepine.

 Download This Application
Download This Application

