Rapid Peptide Separation by Supercritical Fluid Chromatography with Mass Spectrometry
August 18, 2022
Introduction
Interest in supercritical fluid chromatography (SFC) applications is growing due to its ability to reduce analysis times, organic solvent consumption, and waste production. SFC is well established for small molecule pharmaceutical chiral purifications and is continually expanding into other areas. The exploration of aqueous molecules such as peptides was initially inhibited by beliefs that the successful separation of hydrophilic and polar compounds was unlikely by SFC. However, this application note illustrates the ability of SFC to separate peptides. The combination of this high-speed analysis technique with the identification abilities of mass spectrometry provides a versatile system with applicability to a wide variety of the compounds.
Experimental
A JASCO SFC 2000 series was used for the measurement. The system consisted of a PU-2080-CO2 liquefied carbon dioxide delivery pump, PU-2080 HPLC modifier delivery pump, an LV-2080-03 solvent selection valve, an AS-2059-SF autosampler, an HV-2080-06 column switching valve (2), a CO-2060 column oven, an MD-2010 diode array detector, a BP-2080 back-pressure regulator, and Jasco Analyst software.
A 3200 QTRAP LC/MS/MS from Sciex was configured after the back pressure regulator. A connection using peek tubing (0.005” ID) was made directly from the back pressure regulator outlet to the TurboV source of the MS.
The separation column was a Princeton Chromatography 2-Ethylpyrindine column (4.6 mm ID x 250 mm L, 5 mm, 60A). The standard 5 peptide HPLC mixture was purchased from Sigma-Aldrich. It contained G 3502 (GLY-TYR), V 8376 (VAL-TYR-VAL), M 6638 Methionine Enkephalin Acetate (TYR-GLY-GLY-PHE-MET), L 9133 Leucine Enkephalin (TYR-GLY-GLY-PHE-LEU), and A 9525 Angiotensin II Acetate (ASP-ARG-VAL-TYR-ILE-HIS-PRO-PHE) all 0.5 mgs. The sample was dissolved in 1mL of methanol with 0.2% TFA. CO2 with a modifier mobile phase of methanol with 0.2% TFA were used.
Results
Figure 1 shows a SFC chromatogram at 230nm of the standard 5 peptide HPLC mixture. The baseline drift is clearly evident due to the increased absorbance of the methanol/TFA modifier mobile increase over the gradient elution at 230nm. This baseline drift was not seen at 254nm.
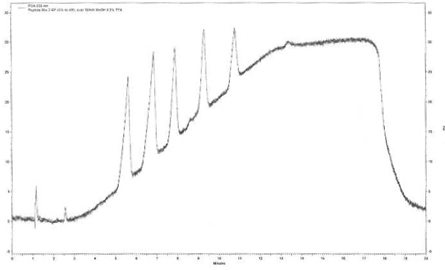
Peaks: 1=V 8376, 2=G 3502, 3=Leucine Enkephalin, 4=Methionine Enkephalin, 5=Angiotension II. Gradient conditions: 30% MeOH 0.2% TFA to 40% MeOH 0.2% TFA over 10 minutes (1%/minute) at 2mLs/minute. 5uLs of 0.5mg/mL peptide mixture was injected.
The separation of this 5 peptide mixture by HPLC required nearly 60 minutes (not including column re-equilibration), compared to less than 12 on SFC. The standard HPLC separation was conducted as a gradient from 5% acetonitrile with 0.1% TFA to 30% acetonitrile with 0.1% TFA over 60 minutes at 1mL/min. These HPLC conditions required 60mLs of liquid (Water-ACN) solvent producing 60mLs of waste compared to 40mLs of mobile phase (CO2-Methanol) and 16mLs of waste (including column re-equilibration) for SFC.
The mass spectrometer was then attached on to the end of the SFC system after the back pressure regulator as described above. The extracted ion chromatogram from the mass spectrometer is shown in figure 2, thus confirming the identities of the peptides.
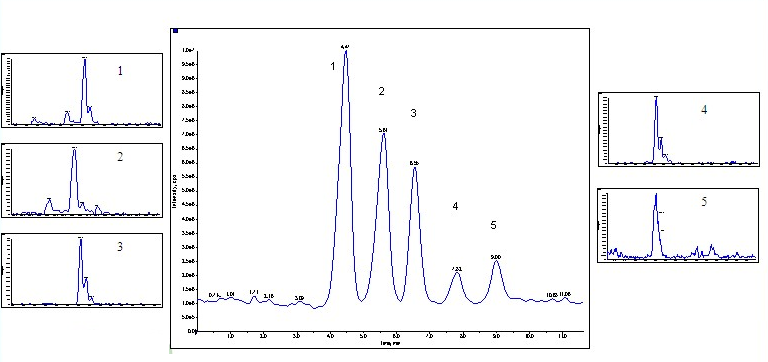
Peaks: 1=V 8376, 2=G 3502, 3=Leucine Enkephalin, 4=Methionine Enkephalin, 5=Angiotension II. Gradient conditions: 30% MeOH 0.2% TFA to 40% MeOH 0.3% TFA over 10 minutes (1%/minute) at 2mLs/minute on Princeton Chromatography 2-ethylpyridine 4.6 x 250mm, 5u column.. 500ngs of each peptide was injected.
The analysis mode on the mass spectrometer was changed to multiple reaction monitoring (MRM) to further identify structural information of the peptides. Figure 3 illustrates the chromatogram of the MRM with formic acid as a mobile phase additive at 0.2% in methanol. Formic acid was used as TFA is known to decrease sensitivity in mass spectrometry, however, as seen significant tailing resulted.
Figure 4 is the MRM chromatogram using TFA as the mobile phase additive producing much sharper peaks even at a lower gradient percentage of modifier. Interestingly enough, the elution order of V8376 and Leucine Enkephalin changes. As expected the sensitivity decreases producing about half the signal to that detected using formic acid.
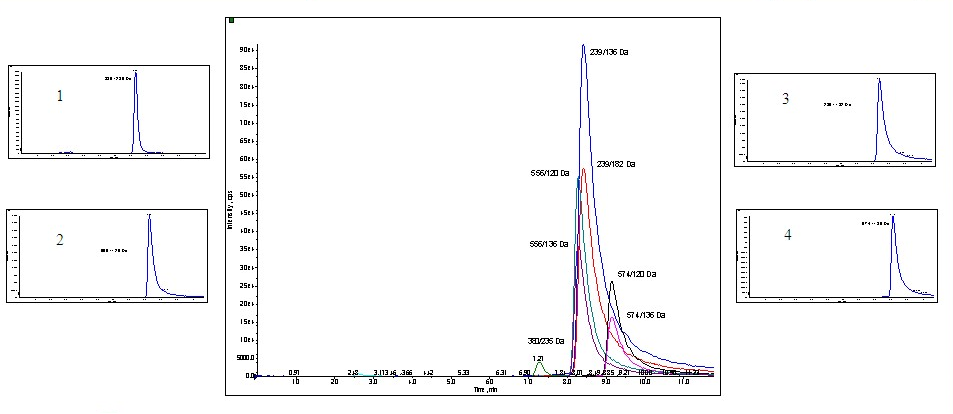
Peaks: Gradient conditions: 20% MeOH 0.2% FA to 40% MeOH 0.2% FA over 10 minutes (1%/minute) at 3mLs/minute on Princeton Chromatography 2-ethylpyridine 4.6 x 250mm, 5u column.. 5ngs of each peptide was injected.
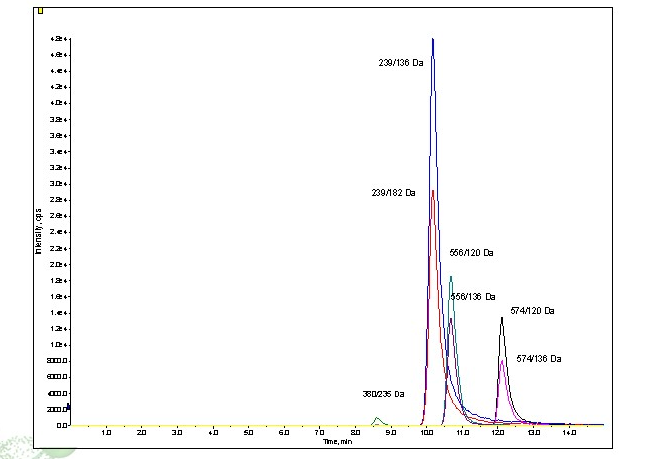
Peaks: Gradient conditions: 20% MeOH 0.2% TFA to 30% MeOH 0.2% TFA over 10 minutes (1%/minute) at 3mLs/minute on Princeton Chromatography 2-ethylpyridine 4.6 x 250mm, 5u column.. 5ngs of each peptide was injected.
The resulting successful separation of a 5 peptide mixture illustrates the ever expanding ability of SFC. The analysis speed is clearly evident in the five fold decrease in analysis time and environmentally friendly nature requiring two-thirds the solvent and producing less than one-third of the organic waste. The combination of SFC with mass spectrometry significantly increased the sensitivity of the technique while also offering identification and structural information of the peptides.
Featured Products:

Rapid Peptide Separation by Supercritical Fluid Chromatography with Mass Spectrometry
Introduction
Interest in supercritical fluid chromatography (SFC) applications is growing due to its ability to reduce analysis times, organic solvent consumption, and waste production. SFC is well established for small molecule pharmaceutical chiral purifications and is continually expanding into other areas. The exploration of aqueous molecules such as peptides was initially inhibited by beliefs that the successful separation of hydrophilic and polar compounds was unlikely by SFC. However, this application note illustrates the ability of SFC to separate peptides. The combination of this high-speed analysis technique with the identification abilities of mass spectrometry provides a versatile system with applicability to a wide variety of the compounds.
Experimental
A JASCO SFC 2000 series was used for the measurement. The system consisted of a PU-2080-CO2 liquefied carbon dioxide delivery pump, PU-2080 HPLC modifier delivery pump, an LV-2080-03 solvent selection valve, an AS-2059-SF autosampler, an HV-2080-06 column switching valve (2), a CO-2060 column oven, an MD-2010 diode array detector, a BP-2080 back-pressure regulator, and Jasco Analyst software.
A 3200 QTRAP LC/MS/MS from Sciex was configured after the back pressure regulator. A connection using peek tubing (0.005” ID) was made directly from the back pressure regulator outlet to the TurboV source of the MS.
The separation column was a Princeton Chromatography 2-Ethylpyrindine column (4.6 mm ID x 250 mm L, 5 mm, 60A). The standard 5 peptide HPLC mixture was purchased from Sigma-Aldrich. It contained G 3502 (GLY-TYR), V 8376 (VAL-TYR-VAL), M 6638 Methionine Enkephalin Acetate (TYR-GLY-GLY-PHE-MET), L 9133 Leucine Enkephalin (TYR-GLY-GLY-PHE-LEU), and A 9525 Angiotensin II Acetate (ASP-ARG-VAL-TYR-ILE-HIS-PRO-PHE) all 0.5 mgs. The sample was dissolved in 1mL of methanol with 0.2% TFA. CO2 with a modifier mobile phase of methanol with 0.2% TFA were used.
Results
Figure 1 shows a SFC chromatogram at 230nm of the standard 5 peptide HPLC mixture. The baseline drift is clearly evident due to the increased absorbance of the methanol/TFA modifier mobile increase over the gradient elution at 230nm. This baseline drift was not seen at 254nm.

Peaks: 1=V 8376, 2=G 3502, 3=Leucine Enkephalin, 4=Methionine Enkephalin, 5=Angiotension II. Gradient conditions: 30% MeOH 0.2% TFA to 40% MeOH 0.2% TFA over 10 minutes (1%/minute) at 2mLs/minute. 5uLs of 0.5mg/mL peptide mixture was injected.
The separation of this 5 peptide mixture by HPLC required nearly 60 minutes (not including column re-equilibration), compared to less than 12 on SFC. The standard HPLC separation was conducted as a gradient from 5% acetonitrile with 0.1% TFA to 30% acetonitrile with 0.1% TFA over 60 minutes at 1mL/min. These HPLC conditions required 60mLs of liquid (Water-ACN) solvent producing 60mLs of waste compared to 40mLs of mobile phase (CO2-Methanol) and 16mLs of waste (including column re-equilibration) for SFC.
The mass spectrometer was then attached on to the end of the SFC system after the back pressure regulator as described above. The extracted ion chromatogram from the mass spectrometer is shown in figure 2, thus confirming the identities of the peptides.

Peaks: 1=V 8376, 2=G 3502, 3=Leucine Enkephalin, 4=Methionine Enkephalin, 5=Angiotension II. Gradient conditions: 30% MeOH 0.2% TFA to 40% MeOH 0.3% TFA over 10 minutes (1%/minute) at 2mLs/minute on Princeton Chromatography 2-ethylpyridine 4.6 x 250mm, 5u column.. 500ngs of each peptide was injected.
The analysis mode on the mass spectrometer was changed to multiple reaction monitoring (MRM) to further identify structural information of the peptides. Figure 3 illustrates the chromatogram of the MRM with formic acid as a mobile phase additive at 0.2% in methanol. Formic acid was used as TFA is known to decrease sensitivity in mass spectrometry, however, as seen significant tailing resulted.
Figure 4 is the MRM chromatogram using TFA as the mobile phase additive producing much sharper peaks even at a lower gradient percentage of modifier. Interestingly enough, the elution order of V8376 and Leucine Enkephalin changes. As expected the sensitivity decreases producing about half the signal to that detected using formic acid.

Peaks: Gradient conditions: 20% MeOH 0.2% FA to 40% MeOH 0.2% FA over 10 minutes (1%/minute) at 3mLs/minute on Princeton Chromatography 2-ethylpyridine 4.6 x 250mm, 5u column.. 5ngs of each peptide was injected.

Peaks: Gradient conditions: 20% MeOH 0.2% TFA to 30% MeOH 0.2% TFA over 10 minutes (1%/minute) at 3mLs/minute on Princeton Chromatography 2-ethylpyridine 4.6 x 250mm, 5u column.. 5ngs of each peptide was injected.
The resulting successful separation of a 5 peptide mixture illustrates the ever expanding ability of SFC. The analysis speed is clearly evident in the five fold decrease in analysis time and environmentally friendly nature requiring two-thirds the solvent and producing less than one-third of the organic waste. The combination of SFC with mass spectrometry significantly increased the sensitivity of the technique while also offering identification and structural information of the peptides.

 Download This Application
Download This Application

