Separation of Basic Drugs by Supercritical Fluid Chromatography
January 8, 2024
Introduction
It is well known that SFC with a column of high polarity material shows the same retention action as normal phase chromatography, and therefore it is believed to be difficult to separate aqueous high polarity components. However, if a little volatile acid, base, or salt is added to modifier solvent(alcohol or etc.), the shape of polar component’s peak can be improved, and components with a long retention time can be eluted with an appropriate retention time.
Various basic drugs were separated using the 2-Ethylpyridine column with ammonium acetatein methanolas modifier solvent.

Experimental

Keywords
747011S
Results
Chromatogram and contour plot of standard mixture of basic drugs (220nm) are shown in Figure 1. As shown, polar components such as Berberine and Maleic were also eluted.
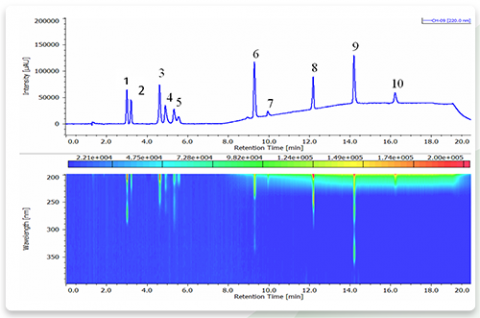
1: Caffeine, 2: Hexobarbital, 3: Amitriptyline, 4: Chlorpheniramine, 5: Imipramine, 6: Quinine, 7:Atropine, 8: Acetaminophen, 9: Berberine,10: Maleic acid
Featured Products:

Separation of Basic Drugs by Supercritical Fluid Chromatography
Introduction
It is well known that SFC with a column of high polarity material shows the same retention action as normal phase chromatography, and therefore it is believed to be difficult to separate aqueous high polarity components. However, if a little volatile acid, base, or salt is added to modifier solvent(alcohol or etc.), the shape of polar component’s peak can be improved, and components with a long retention time can be eluted with an appropriate retention time.
Various basic drugs were separated using the 2-Ethylpyridine column with ammonium acetatein methanolas modifier solvent.

Experimental


Keywords
747011S
Results
Chromatogram and contour plot of standard mixture of basic drugs (220nm) are shown in Figure 1. As shown, polar components such as Berberine and Maleic were also eluted.

1: Caffeine, 2: Hexobarbital, 3: Amitriptyline, 4: Chlorpheniramine, 5: Imipramine, 6: Quinine, 7:Atropine, 8: Acetaminophen, 9: Berberine,10: Maleic acid

 Download This Application
Download This Application

