Supercritical Fluid Extraction of Residual Pesticides in Wheat Flour
August 24, 2022
Introduction
In Japan, on the 29th of May 2006 the Ministry of Health, Labor and Welfare (MHLW) promulgated the Positive List System for residual pesticides, food additives, and veterinary medicines remaining in foods, following the revision of the Food Hygiene Law. In this list approximately 800 kinds of those agricultural chemicals were registered. This system is to prohibit the distribution of foods that contain more than 0.01 ppm of each chemical.
The extraction of residual pesticides in foods has been performed by the solvent extraction method. This method, however, takes about 4 – 5 hours for each extraction, and needs large quantities of organic solvent. In recent years, supercritical fluid extraction (SFE) using supercritical carbon dioxide has attracted much attention as an alternative method to the solvent extraction method.
We have developed a fully automated residual pesticide extraction system, and applied this system to the analysis of a wheat flour sample. Extracted components were analyzed by GC-MS/MS.
Experimental
The newly developed fully automated residual pesticide extraction system was used throughout the experiment. The schematic diagram of this system is shown in Figure 1.
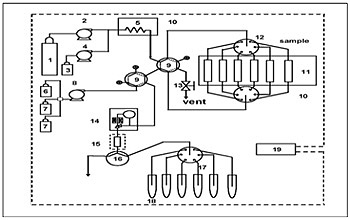
System configuration: 1 = carbon dioxide cylinder, 2 = liquefied carbon dioxide delivery pump, 3 = modifier, 4 = modifier delivery pump, 5 = preheating coil, 6 = solvent for trap elution, 7 = rinse solution for trap column, 8 = solvent delivery pump, 9 = switching valve for flow line, 10 = oven, 11 = extraction vessels, 12 = 6-vessel changer, 13 = release valve, 14 = automatic back pressure regulator, 15 = trap column, 16 = 3-way valve, 17 = 6-way flow line switching valve, 18 = collection tubes, 19 = system controller. Supercritical CO2 delivered by pump 2 passes through one of vessels 11 in which the sample is loaded and then pesticides are extracted. The extracted pesticides are concentrated by a trap column, is eluted by acetonitrile (2 mL) delivered by pump 8, and is collected in one of collection tubes 18. This system is automatically controlled by 19, system controller.
Supercritical fluid extraction conditions:

extraction tube: 10 mL (10 mm x 127 mm), supercritical fluid : CO2, back pressure = 15 MPa, extraction time : 30 min, flow rate : 2mL/min, trap column : ODS (4.6 mm x 50 mm, 30 µm), solvent for trap elution : acetonitrile 2 mL (flow rate : 2mL/min).
As an analytical sample, wheat flour was selected. Sixty eight kinds of pesticides were added to the flour to a concentration of 0.1 ppm for each pesticide except captan, 1 ppm and acetamiprid, 0.5 ppm. Four grams of the wheat flower and 2 g of Hydromatrix (a dehydrating agent) were loaded in each extraction vessel; SFE was applied at an extraction pressure of 15 MPa, at an extraction temperature of 40°C, for an extraction time of 30 min; the extracted components were adsorbed on a trap column; the trapped components were eluted with acetonitrile; the acetonitrile solution was evaporated to dryness with nitrogen gas; and the residue was dissolved in 3 mL of acetone containing 0.05% of PEG200 and PEG400. A portion of this solution was injected onto the GC.
GC Conditions:
Instrument : Quattro micro GC (Waters micromass)
Ionization method : EI
Measurement mode : MRM, SIM
Ionization source temperature : 280°C
Interface temperature : 280°C
GC : 6890N(Agilent)
Injection method : Splitless
Injection volume : 1 µL, Inlet temperature : 250°C
Column : DB-5MS(30 m x 0.25 mm)
Column temperature : 50°C (0 min)—50°C (1 min) —
200°C (7 min) — 250°C (9 min) — 300°C (11 min)
Results
Chromatograms of the standard mixture (upper), the sample added with the standard (middle), and the blank (lower) are shown in Figure 2.
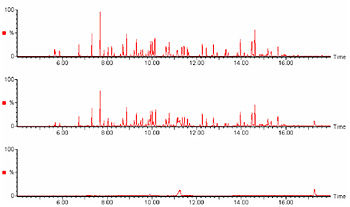
Upper : standard mixture (68 components)
Middle : sample added with standard mixture
Lower : blank Measurement conditions
Standard mixture solution contains 68 components as below.: 1: Acephate, 2: Acetamiprid, 3: Bendiocarb, 4: Bitertanol, 5: Butylate, 6: Captan, 7: Carbaryl 8: Chinomethionat, 9: Chlorfenvinphos, 10: Chlorpyriphos, 11: Cyfluthrin, 12: Cypermethrin, 13: Deltamethrin, 14: Diazinon, 15: Dichlofluanid, 16: Dichlorvos, 17: Diethofencarb, 18: Dimethylvinphos, 19: EPN, 20: Esprocarb, 21: Ethiofencarb, 22: Ethoprophos, 23: Fenarimol, 24: Fenitrothin, 25: Fenobucarb, 26: Fensulfothion, 27: Fenvalerate, 28: Flucythrinate, 29: Flusilazole, 30:Flutolanil, 31: Fluvalinate, 32: Imibenconazole, 33: Iprodione, 34: Isofenphos , 35: Isofenphos P=O, 36: Isoprocarb, 37: Lenacil, 38: Malathion, 39: Mefenacet, 40: Mepronil, 41: Methamidophos, 42: Metolaclior, 43: p,p’-DDE, 44: Paclobutrazol, 45: Pencycuron, 46: Pendimethalin, 47: Permethalin, 48: Phenthoate, 49: Phosalone, 50: Pirimifos-methyl, 51: p,p’-DDD, 52: Pretilachlor, 53: Propiconazole, 54: Pyraclofos, 55: Pyridaben, 56: Pyridaphenthion, 57: Pyrimidifen, 58: Quinalphos, 59: Tefluthrin, 60: Terbucarb, 61: Terbufos, 62: Thenylchlor, 63: Tolclofos-methyl, 64: Triadimenol, 65: a-BHC, 66: ß-BHC, 67: ?-BHC, 68: d-BHC.
As shown in Table 1, among the 68 pesticides, 61 exhibited more than 70% recovery, and 64 more than 50% recovery. The recovery of acetamiprid and methamidophos was as low as 39.4 and 15.6%, respectively. Acetamiprid and methamidophos, due to their high hydrophilicity, indicated low solubility in supercritical carbon dioxide, resulting in a poor recovery in SFE.
| No. | Pesticide | Recovery (%) | No. | Pesticide | Recovery (%) |
|---|---|---|---|---|---|
| 1 | Acephate | 57.1 | 35 | Isofenphos P=O | 90.7 |
| 2 | Acetamiprid | 39.4 | 36 | Isoprocarb | 96.7 |
| 3 | Bendiocarb | 98.7 | 37 | Lenacil | 80.9 |
| 4 | Bitertanol | 91.4 | 38 | Malathion | 94.7 |
| 5 | Butylate | 94.0 | 39 | Mefenacet | 93.3 |
| 6 | Captan | 90.2 | 40 | Mepronil | 97.3 |
| 7 | Carbaryl | 98.6 | 41 | Methamidophos | 15.6 |
| 8 | Chinomethionat | 103.7 | 42 | Metolachlor | 94.4 |
| 9 | Chlorfenvinphos | 108.5 | 43 | p,p'-DDE | 100.0 |
| 10 | Chlorpyriphos | 102.7 | 44 | Paclobutrazol | 87.5 |
| 11 | Cyfluthrin | 105.1 | 45 | Pencycuron | 88.5 |
| 12 | Cypermethrin | 99.0 | 46 | Pendimethalin | 110.8 |
| 13 | Deltamethrin | 85.3 | 47 | Permethalin | 97.8 |
| 14 | Diazinon | 92.5 | 48 | Phenthoate | 102.6 |
| 15 | Dichlofluanid | 85.6 | 49 | Phosalone | 101.0 |
| 16 | Dichlorvos | 87.2 | 50 | Pirimifos-methyl | 97.0 |
| 17 | Diethofencarb | 99.0 | 51 | p,p'-DDD | 101.6 |
| 18 | Dimethylvinphos | 96.8 | 52 | Pretilachlor | 97.5 |
| 19 | EPN | 89.9 | 53 | Propiconazole | 103.0 |
| 20 | Esprocarb | 102.0 | 54 | Pyraclofos | 112.5 |
| 21 | Ethiofencarb | 93.4 | 55 | Pyridaben | 91.9 |
| 22 | Ethoprophos | 100.9 | 56 | Pyridaphenthion | 92.1 |
| 23 | Fenarimol | 117.9 | 57 | Pyrimidifen | 113.2 |
| 24 | Fenitrothion | 110.8 | 58 | Quinalphos | 97.0 |
| 25 | Fenobucarb | 94.8 | 59 | Tefluthrin | 103.7 |
| 26 | Fensulfothion | 95.8 | 60 | Terbucarb | 97.6 |
| 27 | Fenvalerate | 103.7 | 61 | Terbufos | 99.6 |
| 28 | Flucythrinate | 103.9 | 62 | Thenylchlor | 91.0 |
| 29 | Flusilazole | 101.6 | 62 | Tolclofos-methyl | 103.9 |
| 30 | Flutolanil | 96.4 | 64 | Triadimenol | 86.5 |
| 31 | Fluvalinate | 114.4 | 65 | a-BHC | 99.3 |
| 32 | Imibenconazole | 101.0 | 66 | ß-BHC | 96.9 |
| 33 | Iprodione | 109.3 | 67 | Y-BHC | 101.5 |
| 34 | Isofenphos | 99.5 | 68 | d-BHC | 94.6 |
Featured Products:

Supercritical Fluid Extraction of Residual Pesticides in Wheat Flour
Introduction
In Japan, on the 29th of May 2006 the Ministry of Health, Labor and Welfare (MHLW) promulgated the Positive List System for residual pesticides, food additives, and veterinary medicines remaining in foods, following the revision of the Food Hygiene Law. In this list approximately 800 kinds of those agricultural chemicals were registered. This system is to prohibit the distribution of foods that contain more than 0.01 ppm of each chemical.
The extraction of residual pesticides in foods has been performed by the solvent extraction method. This method, however, takes about 4 – 5 hours for each extraction, and needs large quantities of organic solvent. In recent years, supercritical fluid extraction (SFE) using supercritical carbon dioxide has attracted much attention as an alternative method to the solvent extraction method.
We have developed a fully automated residual pesticide extraction system, and applied this system to the analysis of a wheat flour sample. Extracted components were analyzed by GC-MS/MS.
Experimental
The newly developed fully automated residual pesticide extraction system was used throughout the experiment. The schematic diagram of this system is shown in Figure 1.

System configuration: 1 = carbon dioxide cylinder, 2 = liquefied carbon dioxide delivery pump, 3 = modifier, 4 = modifier delivery pump, 5 = preheating coil, 6 = solvent for trap elution, 7 = rinse solution for trap column, 8 = solvent delivery pump, 9 = switching valve for flow line, 10 = oven, 11 = extraction vessels, 12 = 6-vessel changer, 13 = release valve, 14 = automatic back pressure regulator, 15 = trap column, 16 = 3-way valve, 17 = 6-way flow line switching valve, 18 = collection tubes, 19 = system controller. Supercritical CO2 delivered by pump 2 passes through one of vessels 11 in which the sample is loaded and then pesticides are extracted. The extracted pesticides are concentrated by a trap column, is eluted by acetonitrile (2 mL) delivered by pump 8, and is collected in one of collection tubes 18. This system is automatically controlled by 19, system controller.
Supercritical fluid extraction conditions:

extraction tube: 10 mL (10 mm x 127 mm), supercritical fluid : CO2, back pressure = 15 MPa, extraction time : 30 min, flow rate : 2mL/min, trap column : ODS (4.6 mm x 50 mm, 30 µm), solvent for trap elution : acetonitrile 2 mL (flow rate : 2mL/min).
As an analytical sample, wheat flour was selected. Sixty eight kinds of pesticides were added to the flour to a concentration of 0.1 ppm for each pesticide except captan, 1 ppm and acetamiprid, 0.5 ppm. Four grams of the wheat flower and 2 g of Hydromatrix (a dehydrating agent) were loaded in each extraction vessel; SFE was applied at an extraction pressure of 15 MPa, at an extraction temperature of 40°C, for an extraction time of 30 min; the extracted components were adsorbed on a trap column; the trapped components were eluted with acetonitrile; the acetonitrile solution was evaporated to dryness with nitrogen gas; and the residue was dissolved in 3 mL of acetone containing 0.05% of PEG200 and PEG400. A portion of this solution was injected onto the GC.
GC Conditions:
Instrument : Quattro micro GC (Waters micromass)
Ionization method : EI
Measurement mode : MRM, SIM
Ionization source temperature : 280°C
Interface temperature : 280°C
GC : 6890N(Agilent)
Injection method : Splitless
Injection volume : 1 µL, Inlet temperature : 250°C
Column : DB-5MS(30 m x 0.25 mm)
Column temperature : 50°C (0 min)—50°C (1 min) —
200°C (7 min) — 250°C (9 min) — 300°C (11 min)
Results
Chromatograms of the standard mixture (upper), the sample added with the standard (middle), and the blank (lower) are shown in Figure 2.

Upper : standard mixture (68 components)
Middle : sample added with standard mixture
Lower : blank Measurement conditions
Standard mixture solution contains 68 components as below.: 1: Acephate, 2: Acetamiprid, 3: Bendiocarb, 4: Bitertanol, 5: Butylate, 6: Captan, 7: Carbaryl 8: Chinomethionat, 9: Chlorfenvinphos, 10: Chlorpyriphos, 11: Cyfluthrin, 12: Cypermethrin, 13: Deltamethrin, 14: Diazinon, 15: Dichlofluanid, 16: Dichlorvos, 17: Diethofencarb, 18: Dimethylvinphos, 19: EPN, 20: Esprocarb, 21: Ethiofencarb, 22: Ethoprophos, 23: Fenarimol, 24: Fenitrothin, 25: Fenobucarb, 26: Fensulfothion, 27: Fenvalerate, 28: Flucythrinate, 29: Flusilazole, 30:Flutolanil, 31: Fluvalinate, 32: Imibenconazole, 33: Iprodione, 34: Isofenphos , 35: Isofenphos P=O, 36: Isoprocarb, 37: Lenacil, 38: Malathion, 39: Mefenacet, 40: Mepronil, 41: Methamidophos, 42: Metolaclior, 43: p,p’-DDE, 44: Paclobutrazol, 45: Pencycuron, 46: Pendimethalin, 47: Permethalin, 48: Phenthoate, 49: Phosalone, 50: Pirimifos-methyl, 51: p,p’-DDD, 52: Pretilachlor, 53: Propiconazole, 54: Pyraclofos, 55: Pyridaben, 56: Pyridaphenthion, 57: Pyrimidifen, 58: Quinalphos, 59: Tefluthrin, 60: Terbucarb, 61: Terbufos, 62: Thenylchlor, 63: Tolclofos-methyl, 64: Triadimenol, 65: a-BHC, 66: ß-BHC, 67: ?-BHC, 68: d-BHC.
As shown in Table 1, among the 68 pesticides, 61 exhibited more than 70% recovery, and 64 more than 50% recovery. The recovery of acetamiprid and methamidophos was as low as 39.4 and 15.6%, respectively. Acetamiprid and methamidophos, due to their high hydrophilicity, indicated low solubility in supercritical carbon dioxide, resulting in a poor recovery in SFE.
| No. | Pesticide | Recovery (%) | No. | Pesticide | Recovery (%) |
|---|---|---|---|---|---|
| 1 | Acephate | 57.1 | 35 | Isofenphos P=O | 90.7 |
| 2 | Acetamiprid | 39.4 | 36 | Isoprocarb | 96.7 |
| 3 | Bendiocarb | 98.7 | 37 | Lenacil | 80.9 |
| 4 | Bitertanol | 91.4 | 38 | Malathion | 94.7 |
| 5 | Butylate | 94.0 | 39 | Mefenacet | 93.3 |
| 6 | Captan | 90.2 | 40 | Mepronil | 97.3 |
| 7 | Carbaryl | 98.6 | 41 | Methamidophos | 15.6 |
| 8 | Chinomethionat | 103.7 | 42 | Metolachlor | 94.4 |
| 9 | Chlorfenvinphos | 108.5 | 43 | p,p'-DDE | 100.0 |
| 10 | Chlorpyriphos | 102.7 | 44 | Paclobutrazol | 87.5 |
| 11 | Cyfluthrin | 105.1 | 45 | Pencycuron | 88.5 |
| 12 | Cypermethrin | 99.0 | 46 | Pendimethalin | 110.8 |
| 13 | Deltamethrin | 85.3 | 47 | Permethalin | 97.8 |
| 14 | Diazinon | 92.5 | 48 | Phenthoate | 102.6 |
| 15 | Dichlofluanid | 85.6 | 49 | Phosalone | 101.0 |
| 16 | Dichlorvos | 87.2 | 50 | Pirimifos-methyl | 97.0 |
| 17 | Diethofencarb | 99.0 | 51 | p,p'-DDD | 101.6 |
| 18 | Dimethylvinphos | 96.8 | 52 | Pretilachlor | 97.5 |
| 19 | EPN | 89.9 | 53 | Propiconazole | 103.0 |
| 20 | Esprocarb | 102.0 | 54 | Pyraclofos | 112.5 |
| 21 | Ethiofencarb | 93.4 | 55 | Pyridaben | 91.9 |
| 22 | Ethoprophos | 100.9 | 56 | Pyridaphenthion | 92.1 |
| 23 | Fenarimol | 117.9 | 57 | Pyrimidifen | 113.2 |
| 24 | Fenitrothion | 110.8 | 58 | Quinalphos | 97.0 |
| 25 | Fenobucarb | 94.8 | 59 | Tefluthrin | 103.7 |
| 26 | Fensulfothion | 95.8 | 60 | Terbucarb | 97.6 |
| 27 | Fenvalerate | 103.7 | 61 | Terbufos | 99.6 |
| 28 | Flucythrinate | 103.9 | 62 | Thenylchlor | 91.0 |
| 29 | Flusilazole | 101.6 | 62 | Tolclofos-methyl | 103.9 |
| 30 | Flutolanil | 96.4 | 64 | Triadimenol | 86.5 |
| 31 | Fluvalinate | 114.4 | 65 | a-BHC | 99.3 |
| 32 | Imibenconazole | 101.0 | 66 | ß-BHC | 96.9 |
| 33 | Iprodione | 109.3 | 67 | Y-BHC | 101.5 |
| 34 | Isofenphos | 99.5 | 68 | d-BHC | 94.6 |

 Download This Application
Download This Application

