Tianeptine: Assessing Enantiomeric Ratios in Recreationally Abused Products
August 14, 2025Introduction
Abstract
Tianeptine is a synthetic antidepressant that is prescribed in certain parts of the world, but the U.S. Food and Drug Administration does not permit its consumption in the country in any form. The tianeptine molecule has a benzothiazepine core bearing an aminoheptanoate substituent, hence the parts of its non-proprietary name. Its opioid‐like effects have led to widespread nonmedical use under names such as “gas station heroin,” resulting in adverse outcomes and overdoses. Despite its chirality, the enantiomers of tianeptine have not been thoroughly evaluated for their individual therapeutic or toxicological profiles, a gap that depends on access to rapid, reliable chiral separation and quantitation methods. This application note discusses three possible HPLC protocols for the analytical separation of tianeptine’s enantiomers. Two 2.7 μm core–shell silica chiral surface chemistries were employed: a (S, S)-1-(3,5-dinitrobenzamido)-1,2,3,4-tetrahydrophenanthrene phase called the WhelkoShell and a modified macrocyclic glycopeptide phase called the NicoShell. Both columns yielded the same elution order of enantiomers under polar ionic mode, as confirmed by circular dichroism (CD) detection on a JASCO system. Vibrational circular dichroism (VCD) studies assigned the absolute configurations of the purified enantiomers by semi-preparative chromatography. Following the establishment of a simple ethanol-based extraction method for solid and liquid unregulated samples, the total tianeptine content and enantiomeric ratios in illegal recreational and unapproved products were quantified by HPLC-UV absorbance on a JASCO RHPLC-photodiode array (PDA) system.
M. Farooq Wahab, Saba Aslani, Siddharth Jaya Sajeevan J, Daniel W. Armstrong
Department of Chemistry and Biochemistry, University of Texas at Arlington, 76019, USA
Introduction
Depression ranks among the most widespread neurobiological disorders globally, manifesting as persistent difficulties with focus, tiredness, increased anxiety, and consistently low mood. The combination of its widespread impact on health and the absence of a definitive cure has driven extensive pharmacological research and drug-discovery initiatives. Tricyclic antidepressants, such as imipramine, amitriptyline, and protriptyline, demonstrate considerable efficacy in alleviating depressive syndromes, but their use is constrained by marked cardiovascular toxicity. Decades ago, tianeptine was proposed by researchers as a new tricyclic antidepressant with distinctive structural features, a dibenzo-[c,f][1,2]thiazepine S, S-dioxide scaffold, and an aminoheptanoic acid side chain. This structure resulted in mitigating the negative side effects of classical antidepressants.1 Tianeptine has a short half-life and is rapidly eliminated from the body with minimal to no adverse cardiovascular effects; however, there are other safety concerns.1-3 The molecular structure of tianeptine (C21H25ClN2O4S) is shown in Figure 1, and the presence of one chiral carbon leads to a single pair of R/S stereoisomers. Typically, it is sold as a sodium salt.
Unfortunately, the mood-improving benefits come with a potential risk of addiction and misuse, earning tianeptine the nickname of “gas station heroine” from unapproved vendors and unregulated websites.2 The U.S. Food and Drug Administration has made it explicitly clear to the public that tianeptine does not satisfy the Federal Food, Drug, and Cosmetic Act’s definition of a dietary ingredient.4 It is neither a vitamin, mineral, botanical, amino acid, nor any approved dietary substance or derivative, and it likewise lacks approval as a food additive.4 Accordingly, any supplement containing tianeptine is classified as adulterated and constitutes an unsafe food additive. Although tianeptine is prescribed in certain European, Asian, and Latin American countries, it remains unapproved in the United States, where it has been implicated in multiple serious adverse events.4 To date, surprisingly, after decades of its medical use, there is only one comprehensive study of the chiral separation of tianeptine and its actual stereochemical assignment of the R and S isomers.2
Although tianeptine exists as a pair of enantiomers, their distinct therapeutic and toxicological effects remain underexplored, primarily because rapid and dependable methods for their chiral separation and quantification have not been available until recently. In this work, we describe a RHPLC method for the enantioselective separation of tianeptine, employing two 2.7 μm core–shell chiral stationary phases: 1-(3,5-dinitrobenzamido)-1,2,3,4-tetrahydrophenanthrene (WhelkoShell) and a modified macrocyclic glycopeptide column (NicoShell).2,5 By using either normal phase solvents or a polar ionic mobile phase (neat methanol plus ionic modifiers), both columns achieve baseline separation (Rs >1.5) of the R– and S-tianeptine enantiomers in under five minutes, with identical elution order verified by a JASCO circular dichroism (CD) detector.
Experimental
Tianeptine sodium salt (≥95% purity; MW: 458.9 g/mol; CAS: 30123-17-2; Lot: P105-04814) was purchased from ASTA Tech (Bristol, PA, USA). The HPLC columns were provided by AZYP, LLC (TX, USA). The order of elution of tianeptine’s enantiomers was established on two chiral columns, NicoShell (150 mm × 3 mm i.d.) and WhelkoShell (150 mm × 4.6 mm i.d.) by coupling each to a JASCO CD detector (CD-4095) separately. Following auto-zeroing of the CD detector, the baseline CD signal registered an angle of –4.79°. Monitoring was performed at 254 nm, using a detector cell volume of 40 μL. A mobile phase composed of HPLC-grade methanol, acetic acid, and ammonium hydroxide in a volumetric ratio of 100:0.35:0.05 was employed, with a flow rate of 0.425 mL/min for the NicoShell column. For the WhelkoShell column in normal phase, the mobile phase consisted of hexanes and ethanol (50:50 v/v), with a flow rate of 1 mL/min for semi-preparative work, and UV detection at 254 nm (30 μL injections). Mobile phase compositions refer to volume-by-volume (v/v) compositions of solvents. A JASCO RHPLC, equipped with a binary pump (PU-4180) with a degasser and an active mixer, an autosampler (AS-4150) with a 5 μL sample loop, a column oven (CO-4062), and a photodiode array (PDA) detector (MD-4010), was employed for calibration curves and sample chromatograms. A single pump was used after the solvents were manually mixed volume by volume.
Results
Molecular Structure and Absolute Configuration Assignment of Tianeptine
As shown in Figure 1, the core structure consists of a seven-membered sultam (ε-sultam) ring system and a 7-carbon-long carboxylic acid chain.1 A sultam is a cyclic sulfonamide, that is, a ring system in which a sulfonyl group (–SO2–) and an amine nitrogen both reside within the same ring. Structurally, it is the sulfur analog of lactam, whereas a lactam contains a carbonyl (C=O) adjacent to nitrogen; a sultam contains a sulfonyl (S(=O)2) adjacent to nitrogen. X-ray crystallography of the hydrochloride salt tianeptine shows that this sultam ring adopts a boat-type conformation.6 Tianeptine exhibits amphoteric behavior, possessing a carboxylic acid group (pKa ≈ 4.4) and a secondary amine (pKa ≈ 6.86), with a logP of 1.06 at pH 7.4.1 The chiral carbon in the sultam ring is designated by the R or S notation in Figure 1. To assign the absolute configuration, vibrational circular dichroism (VCD) studies were conducted on tianeptine isomers isolated by chiral preparative chromatography.2 A semi-preparative chiral separation in normal phase was performed using a WhelkoShell column (150 mm × 4.6 mm i.d.) with a 50:50 (v/v) ethanol and hexane mobile phase at 1 mL/min. The racemic tianeptine standard was dissolved in the same solvent mixture at ~7.3 mg/mL and injected in 30 μL portions. Collected fractions were manually pooled and then concentrated by rotary evaporation at 25 °C before subsequent configuration analysis.2 VCD is an infrared spectroscopy method that detects the differential absorption of left- versus right-circularly polarized light by optically active molecules. By comparing the experimentally obtained VCD spectrum with spectra calculated from proposed stereochemical models, one can unambiguously assign the molecule’s absolute configuration. For experimental and calculation details, please see the reference.2 JASCO also offers a tutorial on VCD spectrometer features as an application note.7
Chiral Method Development for Tianeptine Enantiomers
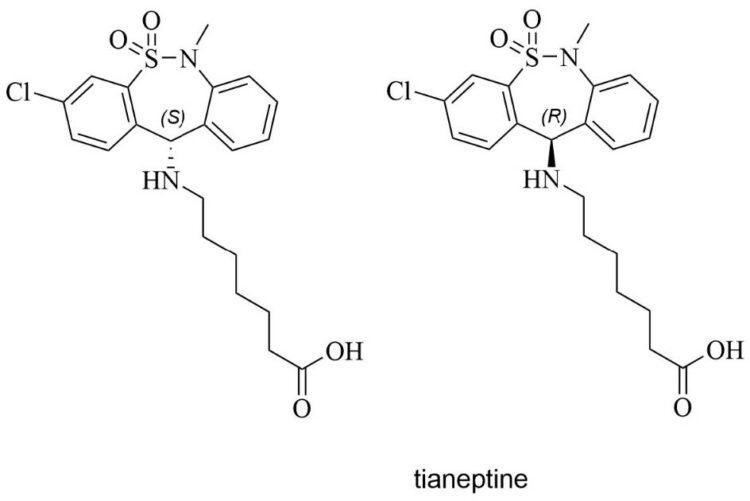
As alluded to in the introduction, there were no published methods for the separation of tianeptine enantiomers in the chemical literature, except for one study.5 A preliminary UV-Vis scan of a tianeptine standard in methanol was acquired to help in the selection of wavelengths in CD and PDA HPLC detectors, in case impurities in the real sample cause interferences. The tianeptine samples, or the gas-station heroin, are usually colored with turmeric additives. The path length of the quartz cuvette was 1 cm. The blank spectra were obtained using pure methanol, as it constitutes the majority of the mobile phase. A 2 mg/mL tianeptine stock solution in methanol was diluted by a ~40-fold and a ~100-fold dilution to obtain the green and the purple spectra in Figure 2A, respectively. Figure 2B shows the expanded inset of the UV-Vis spectrum, with a maximum at 210 nm, followed by a shoulder at 230 nm and a lower-intensity band at 285 nm.
For analytical purposes, two chiral column chemistries with broad selectivity for various classes of molecules were investigated based on the functional groups present in the molecules. A column chemistry that separated the tianeptine enantiomers was the WhelkoShell chemistry (Figure 2C). This stationary phase is usually recommended for normal phase mode; however, herein we demonstrate that polar ionic mode (using neat methanol with additives) also works on the WhelkoShell. The bonded chiral selector is an (S, S)-configured 1-(3,5-dinitrobenzamido)-1,2,3,4-tetrahydrophenanthrene, whose cleft-shaped pocket is crucial for enantioselectivity. The second column chemistry is the NicoShell, a proprietary macrocyclic glycopeptide5 bonded to superficially porous silica particles, which exhibits good selectivity for basic compounds. Because tianeptine contains ionizable functionalities (a secondary amine and a carboxylic acid) and can engage in hydrogen bonding, its enantiomers were resolved on a NicoShell column under polar ionic conditions in the presence of acetic acid and ammonium hydroxide as mobile phase modifiers, as shown in Figure 2D. Since the CD detector cell volume is 40 μL, the extra-column effect slightly lowers the resolution; however, the goal was to confirm the opposite directions (positive and negative peaks) of CD signals for tianeptine standards (Figure 2E–F). This resolution compromise with a large cell is no longer a problem because peak modeling can readily extract the true peak profile, even if there is mild overlap.8 The CD detector confirms that separate column chemistries and different eluent systems yield the same elution order of tianeptine enantiomers. Further analytical work was done on the NicoShell column in polar ionic mode.
Tianeptine Extraction Approach from Real Samples
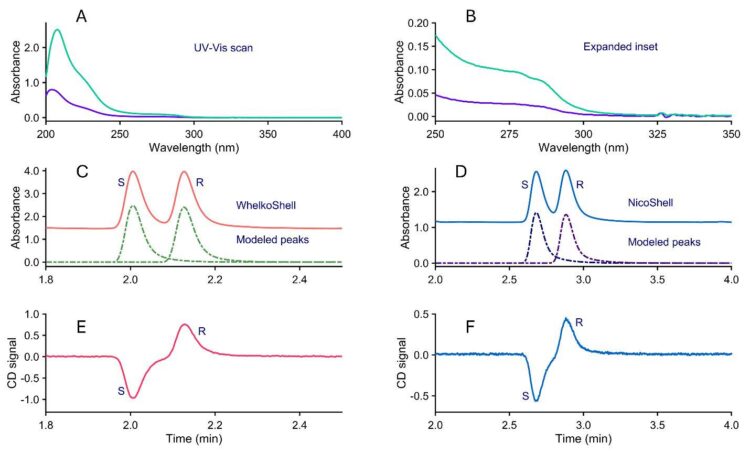
Unapproved vendors sell tianeptine in the form of powder-filled capsules and paste, which contain a significant number of undisclosed substances, with turmeric powder serving as another major additive. These unknown components can appear as unwanted peaks near the actual tianeptine enantiomers. A key advantage of using polar ionic mode is that methanol, being a strong solvent, elutes most of the undesirable impurities at the dead time on the NicoShell column. Another question during this study was how to effectively extract all the drug from these powdery or oily complex matrices.
For the solid product, about 30 mg of the capsule contents was transferred into a 1.5 mL Eppendorf tube. The sample was extracted five times by adding 1 mL of ethanol, sonicating for 30 seconds, centrifuging at 13,000 rpm for 5 minutes, and carefully decanting the supernatant each time. The reason for choosing the 5-step extraction becomes clear in Figure 3, where three extractions are sufficient, but to ensure completion, five extractions are conducted. The combined extracts were then concentrated by rotary evaporation at 25 °C, and the residue was brought up to 1 mL with ethanol in Class A 1 mL volumetric flasks. After filtration, the solution was injected into the HPLC. For the liquid product, a nominal 300 μL was pipetted into a vial and weighed. Then, 2 mL of ethanol was added, and the mixture was shaken and filtered through a 0.22 μm syringe filter. The filter was washed with 1 mL of ethanol, which was discarded after filtration, and 3 mL of ethanol, which was retained after filtration. The vial was also rinsed with ethanol, which was kept. The final rinses were to ensure all the tianeptine was quantitatively transferred. The filtrate and the collected ethanol were mixed and then rotary evaporated to reduce the volume. The volume was adjusted to 1 mL using ethanol in a Class A volumetric flask.
Calibration and Quantitation using JASCO’s RHPLC with PDA Detection
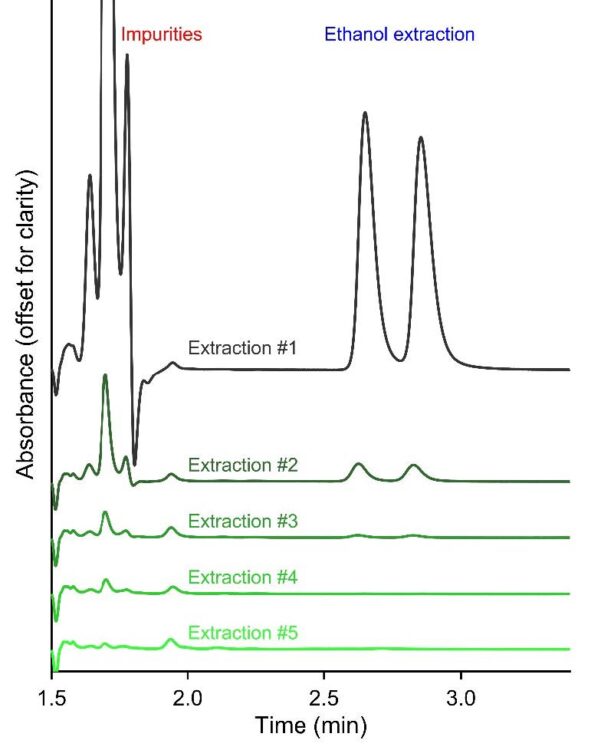
For quantitative purposes, tianeptine standards were prepared in a range that bracketed the expected concentration of the sample solutions (0.3 to 10 mM). The absorption maximum of tianeptine is at 210 nm. Unfortunately, most organic compounds and solvents will start absorbing at this wavelength, so we selected the 220 nm channel from the PDA data. Higher concentrations, such as 15 or 20 mM tianeptine at 210 nm, started to show non-linearity in the calibration curve. Deviations from linearity arise fundamentally in an absorbance–concentration calibration curve whenever one or more of the Beer–Lambert law’s underlying assumptions break down. At elevated concentrations, the sample itself begins to attenuate the incident beam so strongly that the light intensity reaching deeper layers is substantially reduced. This “inner-filter” phenomenon causes the apparent absorption per unit concentration to drop off, resulting in a concave-downward calibration curve. With PDA data, any wavelength as shown in the UV spectra can be conveniently selected. Figure 4 shows the overlaid chromatograms, as well as the linear region of the calibration curve at 220 nm, with six standards, yielding an R2 value of 0.9995 and an equation Peak Area = 915190.04 C + 96754.26, where C is the concentration of total tianeptine stereoisomers in mM.
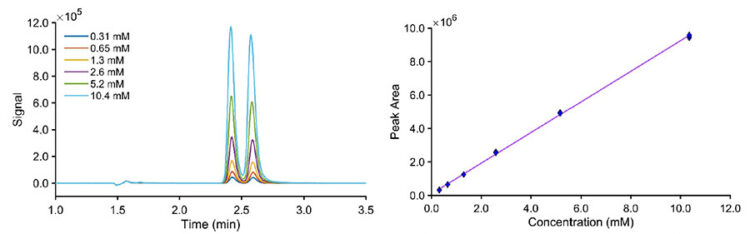
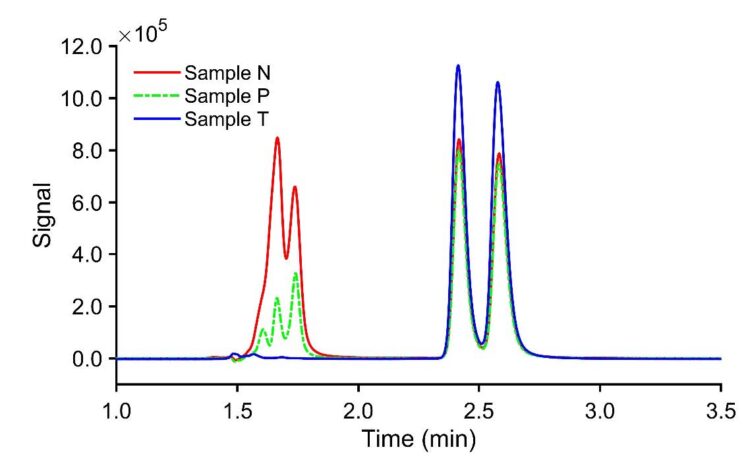
Unregulated samples from vendors exhibited a racemic composition of tianeptine after ethanol extraction, as confirmed by RHPLC analysis on the JASCO system, as shown in Figure 5. Sample N was a syrupy liquid, and samples P and T were powders inside tablets. The range of total tianeptine in these products was found to be 49 – 55 mg per tablet, and the liquid bottle contained approximately 110 mg of total tianeptine. In enantiomeric analysis, one can perform total area calibration as shown in this application note. From the chiral analysis, we can assess the enantiomeric ratios. This technique enables the rapid determination of the fractional mass of each enantiomer when needed.
Conclusion
Tianeptine enantiomers were conveniently resolved in polar ionic mode on both NicoShell and WhelkoShell columns, although the latter column chemistry functions primarily in normal phase mode. Preparative HPLC allowed isolation of each isomer, which were then characterized by VCD spectra; the first peak corresponded to the S enantiomer, and the second to the R enantiomer. The JASCO CD detector enables us to confirm the elution order of the stereoisomers, which is not possible using UV absorbance or even mass spectrometry. Analysis of unregulated dietary supplements revealed tianeptine levels two to eight times higher than the recommended adult daily dose, yet none of the products exhibited any significant enantiomeric excess.
References
1. Nishio, Y.; Lindsley, C. W.; Bender, A. M. Classics in Chemical Neuroscience: Tianeptine. ACS Chemical Neuroscience 2024, 15 (21), 3863-3873.
2. Aslani, S.; Nafie, J.; Wahab, M. F.; Armstrong, D. W. Tianeptine: enantiomeric separations, structural assignment, and biological interactions. Talanta 2025, 294, 128197.
3. Juvent, M.; Douchamps, J.; Delcourt, E.; Kostucki, W.; Dulcire, C.; d’Hooge, D.; Herchuelz, A. Lack of Cardiovascular Side Effects of the New Tricyclic Antidepressant Tianeptine A Double-Blind, Placebo-Controlled Study in Young Healthy Volunteers. Clinical neuropharmacology 1990, 13 (1), 48-57.
4. US Food and Drug Administration: Tianeptine in Dietary Supplements. 2025. https://www.fda.gov/food/information-select-dietary-supplement-ingredients-and-other-substances/tianeptine-dietary-supplements (Accessed July 9, 2025)
5. Aslani, S.; Wahab, M. F.; Kenari, M. E.; Berthod, A.; Armstrong, D. W. An examination of the effects of water on normal phase enantioseparations. Analytica Chimica Acta 2022, 1200, 339608.
6. Mishnev, A.; Zvirgzdins, A.; Actins, A.; Delina, M. 7-[(3-Chloro-6-methyl-6, 11-dihydrodibenzo [c, f][1, 2] thiazepin-11-yl) amino] heptanoic acid S, S-dioxide hydrochloride. Structure Reports 2012, 68 (11), o3136-o3136.
7. JASCO Application Note: Vibrational Circular Dichroism Spectroscopy using an FVS-6000 Spectrometer. 2025. https://jascoinc.com/applications/measurement-of-vibrational-circular-dichroism-spectra/
8. Burk, R. J.; Wahab, M. F.; Armstrong, D. W. Influence of theoretical and semi-empirical peak models on the efficiency calculation in chiral chromatography. Talanta 2024, 277, 126308.

 Download This Application
Download This Application