Water Analysis for Aldehydes using Post-Column Derivatization by HPLC
January 8, 2024
Introduction
It is a growing concern that aldehydes such as formaldehyde and acetaldehyde may pollute air and water in lakes, reservoirs, and rivers in the environment. Therefore, the aldehydes are subjected to regulations by Air Pollution Control, Water Supply and Offensive Odor Control Acts in Japan.
Pre-column derivatization with 2,4-DNPH is known as a method to measure aldehydes in HPLC, however, the pretreatment of samples such as collection then condensation or extraction is required. JASCO introduced a method for the analysis of formaldehyde and acetaldehyde in water by post-column fluorescence derivatization using 1,3- Cyclohexanedione as derivatization reagent which did not require such pretreatment.
In this report, we extended the applicability of the method to other aldehydes by optimizing the derivatization conditions.

Experimental
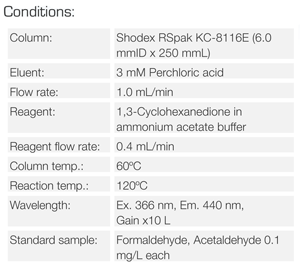
Keywords
820022H
Results
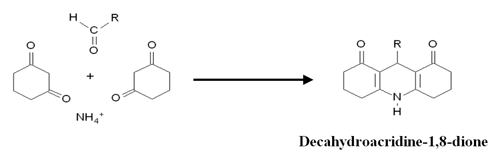
Figure 1 shows the chemical reaction of 1,3-Cyclohexanedione with aldehydes during the post-column derivatization and figure 2 shows flow path of the system.
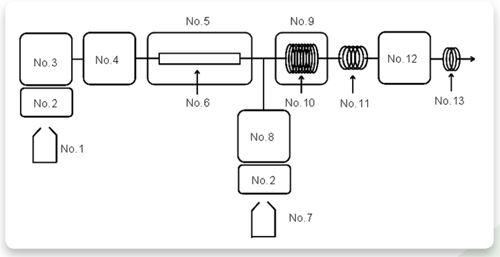
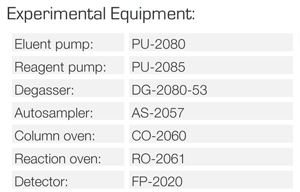
No.1 : Eluent
No.2 : Degasser (DG-2080-53)
No.3 : Pump for eluent (PU-2080)
No.4 : Autosampler (AS-2057)
No.5 : Column oven (CO-2060)
No.6 : Column (Shodex RSpak KC-811 6E)
No.7 : Reagent
No.8 : Pump for reagent (PU-2085)
No.9 : Reaction oven (RO-2061)
No.10: Reaction coil
No.11: Cooling coil
No.12: Fluorescence detector (FP-2020)
No.13: Backpressure coil
Figure 3 shows chromatogram of the standard mixture of formaldehyde and acetaldehyde. The two components were separated within 8 minutes.
Figures 4, 5 and 6 show chromatograms of drinking water, river water and rain water, respectively. The top chromatograms are of neat samples and the bottom ones are of samples spiked by adding 0.1 mg/L of formaldehyde and acetaldehyde. All of the samples were separated.
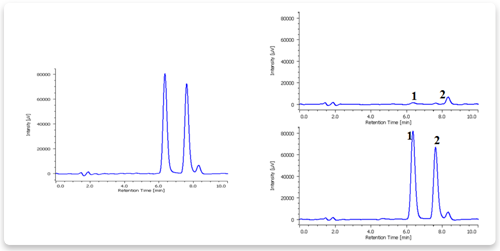
Figure 4. Chromatogram of drinking water.
1: Formaldehyde, 2: Acetaldehyde
Preparation. Drinking water was filtered using 0.45 µm membrane filter.
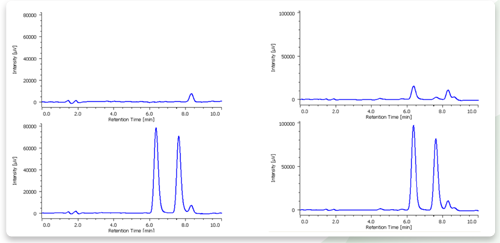
1: Formaldehyde, 2: Acetaldehyde
Preparation. River water was filtered with 0.45 µm membrane filter.
Figure 6. Chromatogram of rain water.
1: Formaldehyde, 2: Acetaldehyde
Preparation: Rain water was filtered with 0.45 µm membrane filter.
Featured Products:

Water Analysis for Aldehydes using Post-Column Derivatization by HPLC
Introduction
It is a growing concern that aldehydes such as formaldehyde and acetaldehyde may pollute air and water in lakes, reservoirs, and rivers in the environment. Therefore, the aldehydes are subjected to regulations by Air Pollution Control, Water Supply and Offensive Odor Control Acts in Japan.
Pre-column derivatization with 2,4-DNPH is known as a method to measure aldehydes in HPLC, however, the pretreatment of samples such as collection then condensation or extraction is required. JASCO introduced a method for the analysis of formaldehyde and acetaldehyde in water by post-column fluorescence derivatization using 1,3- Cyclohexanedione as derivatization reagent which did not require such pretreatment.
In this report, we extended the applicability of the method to other aldehydes by optimizing the derivatization conditions.

Experimental


Keywords
820022H
Results
Figure 1 shows the chemical reaction of 1,3-Cyclohexanedione with aldehydes during the post-column derivatization and figure 2 shows flow path of the system.


No.1 : Eluent
No.2 : Degasser (DG-2080-53)
No.3 : Pump for eluent (PU-2080)
No.4 : Autosampler (AS-2057)
No.5 : Column oven (CO-2060)
No.6 : Column (Shodex RSpak KC-811 6E)
No.7 : Reagent
No.8 : Pump for reagent (PU-2085)
No.9 : Reaction oven (RO-2061)
No.10: Reaction coil
No.11: Cooling coil
No.12: Fluorescence detector (FP-2020)
No.13: Backpressure coil
Figure 3 shows chromatogram of the standard mixture of formaldehyde and acetaldehyde. The two components were separated within 8 minutes.
Figures 4, 5 and 6 show chromatograms of drinking water, river water and rain water, respectively. The top chromatograms are of neat samples and the bottom ones are of samples spiked by adding 0.1 mg/L of formaldehyde and acetaldehyde. All of the samples were separated.

Figure 4. Chromatogram of drinking water.
1: Formaldehyde, 2: Acetaldehyde
Preparation. Drinking water was filtered using 0.45 µm membrane filter.

1: Formaldehyde, 2: Acetaldehyde
Preparation. River water was filtered with 0.45 µm membrane filter.
Figure 6. Chromatogram of rain water.
1: Formaldehyde, 2: Acetaldehyde
Preparation: Rain water was filtered with 0.45 µm membrane filter.

 Download This Application
Download This Application

