Principals of Vibrational Circular Dichroism
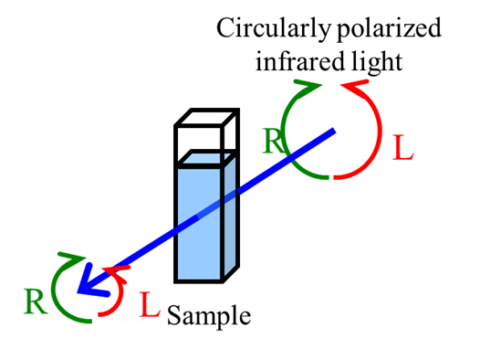
Vibrational circular dichroism (VCD) is a spectroscopy technique used to measure the absorption difference between left-handed and right-handed circularly polarized light in the infrared region. This is distinguished from electronic circular dichroism (ECD or CD), which focuses on the ultraviolet region.
Substances that exhibit CD are called optically active compounds. They have enantiomers that cannot be superimposed on their mirror image, as is the case of human hands. The example shown here is for alanine.
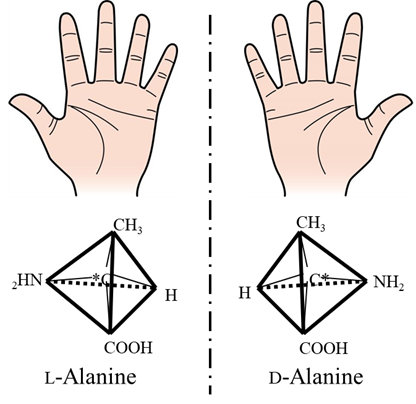
In addition to point chirality involving asymmetric carbon atoms as in the case of alanine, optically active compounds can also exhibit planar chirality involving their front and back sides, axial chirality associated with rotation about a particular axis, and helical chirality associated with helical structures.
Information that can be obtained from VCD, First, since all organic compounds absorb in the infrared region, there is no need to add a chromophore to the sample.
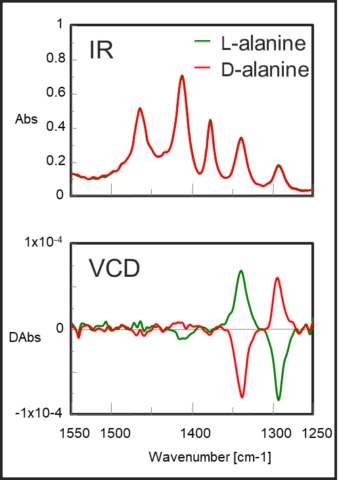
IR and VCD spectra of L – alanine and D – alanine
In addition to the identification of chiral compounds, a big advantage is that the absolute configuration can be determined by comparing the measured spectrum with that based on molecular orbital calculations
However, there are some disadvantages associated with VCD. The first is that a high sample concentration is needed, implying a large amount of sample. The second is that VCD signals are very weak. In fact, the signal intensity is more than two orders of magnitude lower than that for ECD, and measurement can take more than one hour. As a consequence, the sensitivity and stability of the instrument are of primary importance.
Instrumentation of Vibrational Circular Dichroism
VCD instrument uses an FTIR bench, with a Michelson interferometer. VCD can be a dedicated instrument or attachment accessory. A ceramic light source is used as an infrared source, polarization optics, and highly sensitive detector. The Interferometer has an angle of incidence of 28º increasing the sensitivity of the instrument. This is a high throughput FTIR.
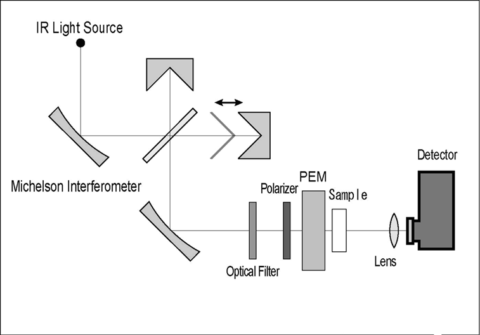
The detector used in the JASCO VCD instrument is a photovoltaic mercury cadmium telluride (PV-MCT) detector, which is a highly sensitive detector and does not become saturated even if no metal mesh is used, so there is no loss of sensitivity. In fact, the sensitivity is about three to four times higher than that for a photoconductive MCT (PC-MCT).
Stability
A ceramic light source is used as an infrared source and is located outside in an isolated mount from the optical bench and main cover to reduce the thermal drift. This light is then collimated and sent to a polarizer and continues to a photoelastic modulator (PEM), the combination of these elements reduces the creation of artifacts. Thermostatted blocks in the interferometer and the PEM maintain a constant temperature inside the instrument avoiding any perturbation in the data.
Vibrational Circular Dichroism Sample Preparation
For VCD measurements are made using IR transmission method, a liquid sample cell is used. Two types of cell materials window plates are used, barium fluoride and calcium fluoride. Barium fluoride window transmits light up to 800 cm-1 and is slightly soluble in water. On the other hand, the calcium fluoride plate transmits light up to 1100 cm-1 and is insoluble in water. So, the calcium fluoride plate can be used with aqueous solution samples and the barium fluoride plate can be used with non-aqueous solution samples.

Solvents
In VCD measurements, better results are obtained with a solvent with less IR absorption. If light absorption by the solvent is too large, the detector will not be able to detect light from the sample. Therefore, for VCD measurements, carbon tetrachloride, chloroform and dichloromethane are better because they absorb only weakly in the infrared region.
Also, since solvents such as hexane, toluene and others have absorption bands associated with–CH bonds, the sample cannot be measured in this region. For water, IR light is absorbed in almost all wavenumbers.
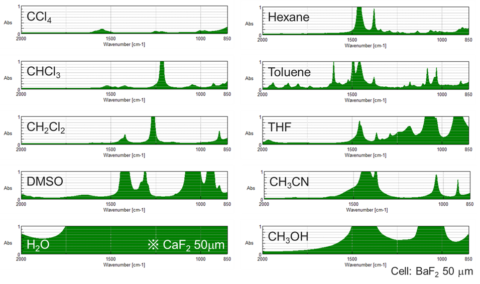
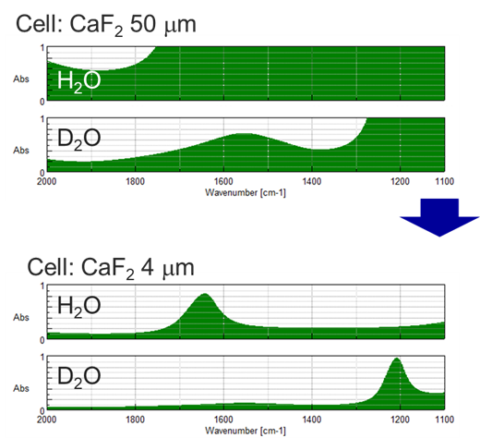
In considering the case for water. The use of heavy water decreases solvent absorption, but it still remains strong. Optical path length can be shortened to reduce the amount of absorption. However, a higher concentration sample will be required.
Vibrational Circular Dichroism Applications
The main application for VCD instrumentation is the absolute configuration of chiral organic molecules. Analysis usually starts with the simulation of infrared spectrum using the dipole moment of molecules, or enthalpy energy of bonds. Then calculated VCD spectrum optimized based on the molecular structure, after VCD spectrum is measured, calculated and measured VCD spectra are compared and configuration is determined .
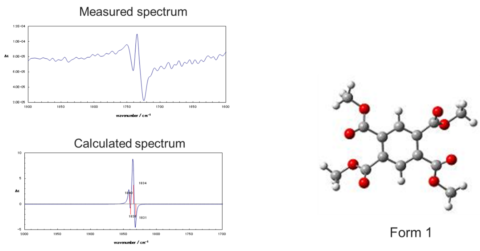
L-carnitine is the nutrient used when converting fat into energy and it is used for diet foods and food additives. On the other hand, since D-carnitine is toxic, the synthesis of enantiopure carnitine and analysis of excess enantiomer percentage is important.
Analysis of carnitine by ECD and optical rotation has been reported, but there are almost no examples of VCD measurements that obtained useful information. Measurements of the carnitine family members such as carnitine nitrile chloride and estimation of the conformation were calculated.
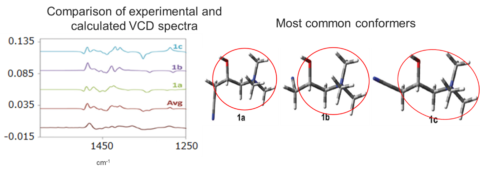
VCD spectrum measurements were performed for carnitine nitrile chloride, stable conformations were calculated, and a comparison was made between the experimental and calculation results.
These three conformations were found to be the most stable for this molecule and it is assumed that all parts except the CH2CN group had a rigid structure. Since the information obtained from the VCD spectrum is abundant, in addition to chirality recognition, determination of absolute configuration and examination of conformational characteristics can be performed.
Vibrational Circular Dichroism Webinar
References

