Achiral Preparative Separation Using a Preparative Supercritical Fluid Chromatography (SFC) System and a Gas-Liquid Separation Nozzle (MCS-es)
October 21, 2024
Introduction
Preparative separation utilizing Supercritical Fluid Chromatography (SFC) offers several distinctive advantages over traditional chromatography methods. SFC enables higher throughput compared to High-Performance Liquid Chromatography (HPLC). The primary solvent of the mobile phase, supercritical carbon dioxide (CO2), transitions to a gas upon recovery, simplifying post-processing steps, such as evaporation and drying procedures. Furthermore, CO2 is more cost-effective compared to organic solvents and can be sourced in high purity (>99.99%). On the other hand, CO2 experiences the Joule-Thomson cooling effect and undergoes an adiabatic expansion approximately 500 times to 1 when it is exposed to the lower atmospheric pressure at the outlet of the back pressure regulator. Consequently, during preparative separation, it is necessary to take measures to prevent contamination, such as condensed water from sample scattering and sudden temperature drops.
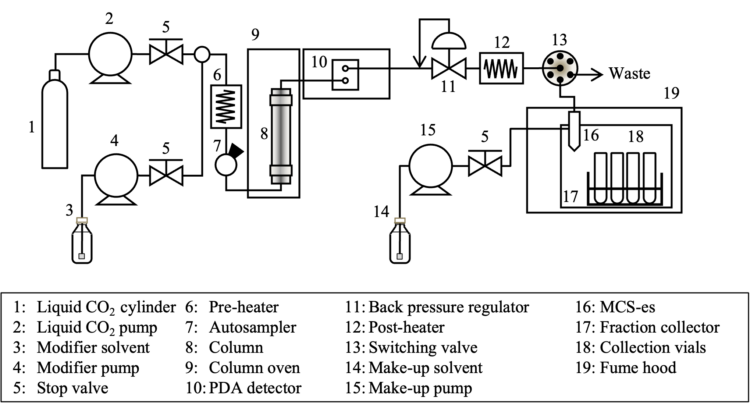
In this application note, it is demonstrated how the installation of the MCS-es, a gas-liquid separation nozzle, on the Z-axis arm of the Gilson 223 Sample Changer fraction collector facilitates the gradual collection of only the liquid phase. This innovative setup effectively minimizes scattering and condensation due to CO2 expansion, enabling efficient preparative separation with high recovery rates across a wide range of flow rates. In addition, it allows for the direct incorporation of make-up solvent into the MCS-es (patent pending). The achiral separation of a standard solution containing three components (1,000 µg/mL caffeine, 500 µg/mL anthracene, and 500 µg/mL fluorene in methanol) was performed using the LC-4000 Series Preparative SFC system in conjunction with the Gilson 223 Sample Chamber fraction collector equipped with the MCS–es.
Experimental
SFC System Configuration
| Liquid CO2 Pump | PU-4388 | Back Pressure Regulator (BPR) | BP-4340 |
| Modifier Pump | PU-4088* | Post-heater | HE-02 |
| Pre-heater | HE-01 | Heater Controller | HC-4068-01 |
| Heater Controller | HC-4068-01 | Make-up Pump | PU-4086* |
| Autosampler | AS-4358 | Fraction Valve | FV-4000-06 |
| Column Oven | CO-4060* | Fraction Collector | Gilson 223 Sample Chamber |
| Photo Diode Array (PDA) Detector | MD-4010* | Fume Hood | FH-4388 |
| *equipped with optional units | |||
SFC Conditions
| Column | YMC-Pack Pro C18 (30 mm I.D. x 250 mm L, 10 µm) |
| Mobile Phase (%) | Supercritical carbon dioxide (CO2)/Methanol (MeOH) (95/5) |
| Flow Rate | 120 mL/min |
| Column Temperature | 40 ºC |
| Pre-heat Temperature | 40 ºC |
| Wavelength | 200 – 650 nm; 215 nm (CH1), 270 nm (CH9), 258 nm (CH10), and 244 nm (CH11) |
| Back Pressure | 10 MPa |
| Post-heat Temperature | 60 ºC |
| Make-up Solvent | Methanol |
| Make-up Solvent Flow Rate | 10 mL/minute |
| Injection Volume | 1,000 µL |
| Standard Solution | 1,000 µg/mL caffeine, 500 µg/mL anthracene, and 500 µg/mL fluorene in methanol |
SFC Schematic Diagram
Keywords
Preparative Supercritical Fluid Chromatography, Preparative SFC, Prep Supercritical Fluid Chromatography, Prep SFC, supercritical carbon dioxide, supercritical CO2, gas-liquid separator, MCS-es, fraction collector, Gilson 223 Sample Changer, YMC-Pack Pro C18, achiral separation, achiral preparation
Results
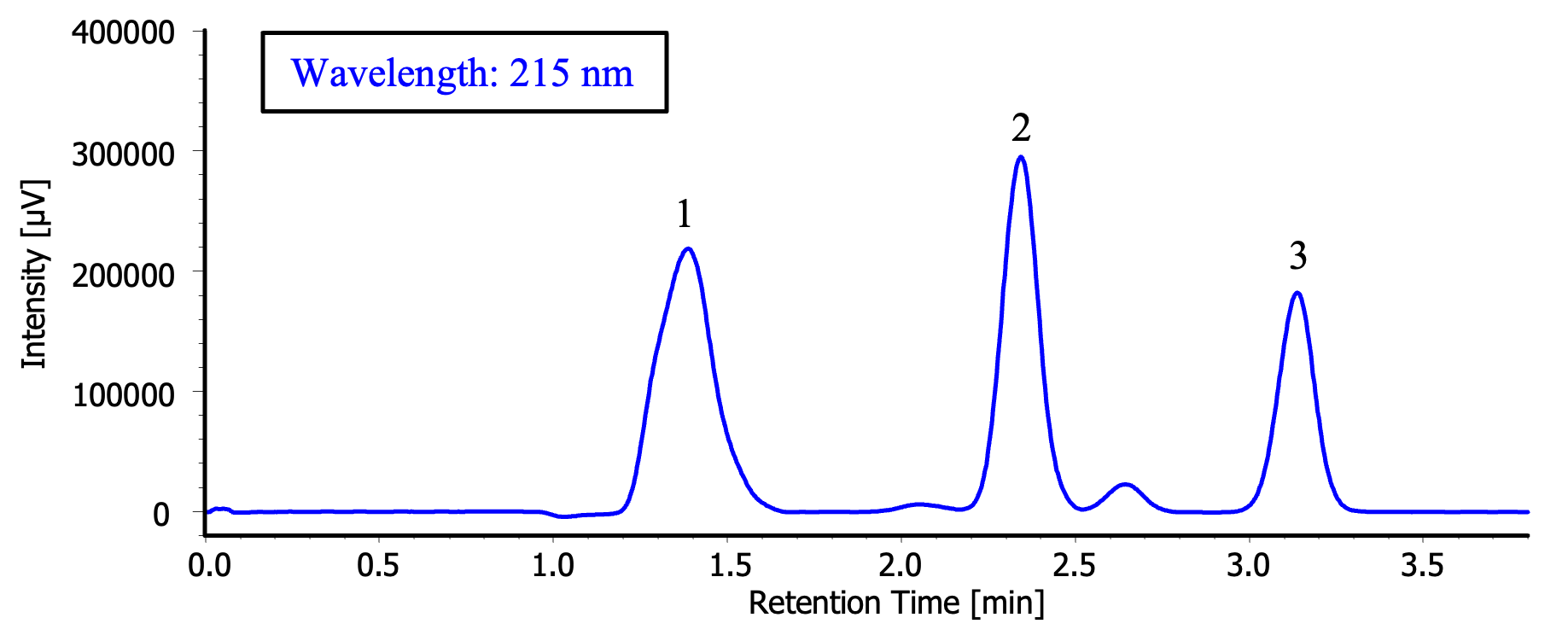
1: Caffeine, 2: Anthracene, 3: Fluorene
Figure 1 shows the chromatogram of the standard solution containing three components (1,000 µg/mL caffeine, 500 µg/mL anthracene, and 500 µg/mL fluorene in methanol). Based on this chromatogram, a fraction collection method was created and used for the preparative separation of the standard solution.
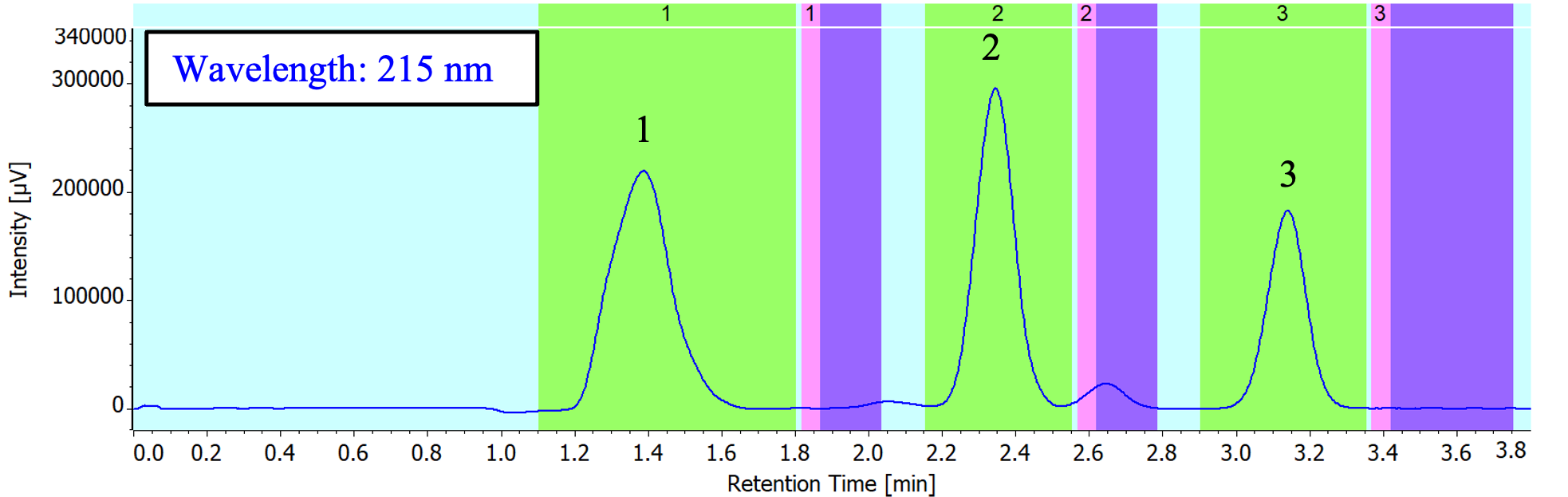
1: Caffeine, 2: Anthracene, 3: Fluorene
Figure 2 shows the fraction collection results from the achiral preparative separation of the standard solution containing three components (caffeine, anthracene, and fluorene) based on time fractionation. In Figure 2, the green section represents the collection phase, the pink section represents the make-up phase, and the purple section represents the drop waiting time*1.
*1 The time taken to collect the modifier and make-up solvent remaining in the MCS-es after valve switching.
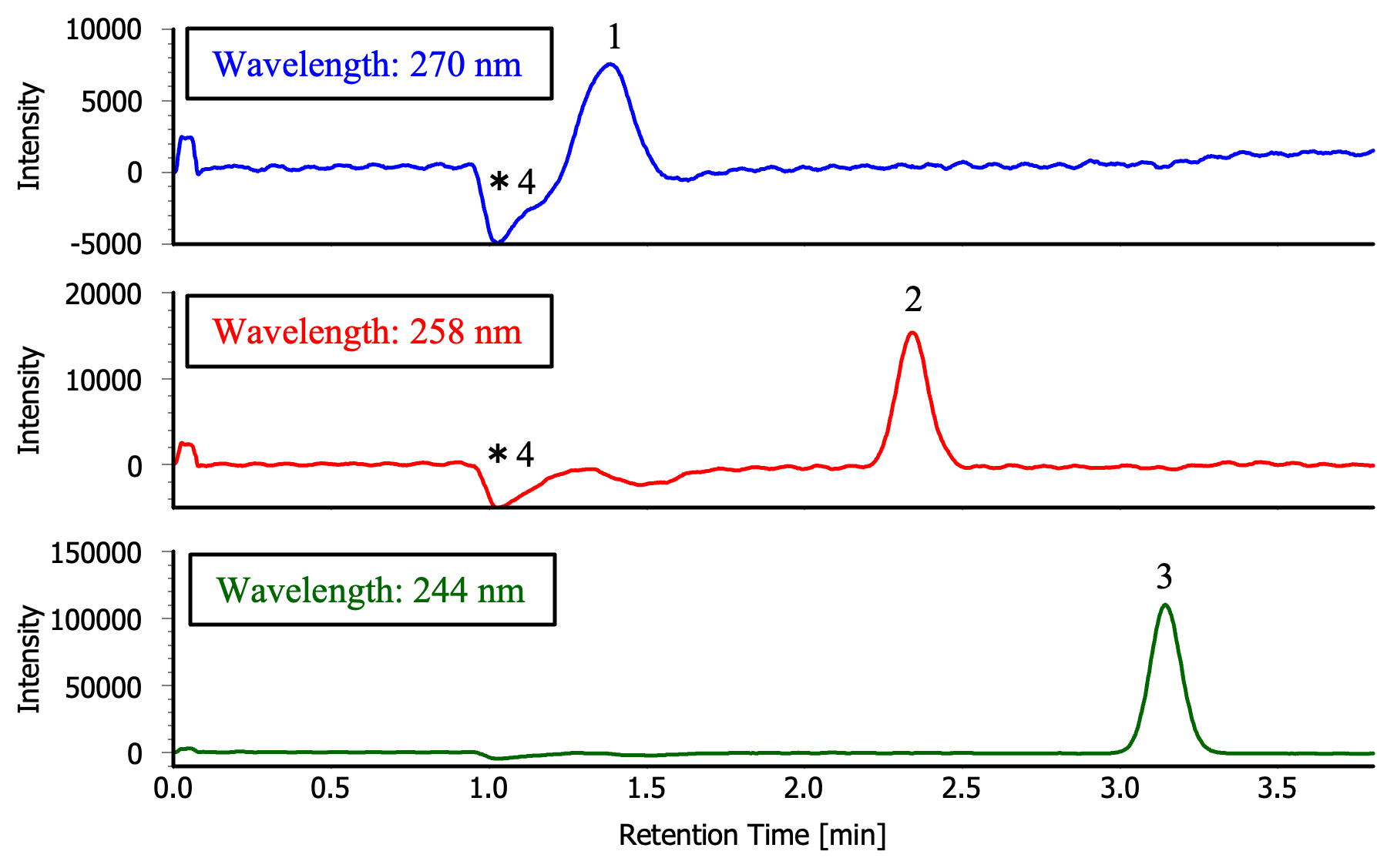
1: Caffeine (equivalent to 50 µg/mL),
2: Anthracene (equivalent to 25 µg/mL),
3: Fluorene (equivalent to 25 µg/mL),
*4: Solvent peak
Figure 3 shows the chromatograms obtained by re-measuring each fraction that was collected in Figure 2. Each fraction was diluted to 20 mL by co-washing with methanol so that caffeine, anthracene, and fluorene were each 50 µg/mL, 25 µg/mL, and 25 µg/mL, respectively (equivalent). These solutions were used for recovery rate measurements.
Table 1 shows the recovery rates for each component of the standard solution, calculated assuming the area values at the time of measurement of the standard solution as 100% (50 µg/mL caffeine, 25 µg/mL anthracene, and 25 µg/mL fluorene). Excellent recovery rates were obtained for all components.
Table 1. Recovery Rates of the Three Components (1: Caffeine, 2: Anthracene, 3: Fluorene) in the Standard Solution
| Caffeine | Anthracene | Fluorene | |
| Recovery Rate % | 98.06 | 94.82 | 93.57 |
Conclusion
The use of the Preparative SFC system in conjunction with the Gilson 223 Sample Chamber fraction collector equipped with MCS-es gas-liquid separation nozzle successfully achieved achiral separation and preparation of a standard solution containing 3 components (caffeine, anthracene, and fluorene) with excellent recovery rates.
The MCS-es can be used with a maximum total flow rate of up to 200 mL/min (under specified conditions). It also supports preparation at relatively low flow rates on an analytical scale, so it can be applied to preparation over a wide range of flow rates.
Related Products

Achiral Preparative Separation Using a Preparative Supercritical Fluid Chromatography (SFC) System and a Gas-Liquid Separation Nozzle (MCS-es)
Introduction
Preparative separation utilizing Supercritical Fluid Chromatography (SFC) offers several distinctive advantages over traditional chromatography methods. SFC enables higher throughput compared to High-Performance Liquid Chromatography (HPLC). The primary solvent of the mobile phase, supercritical carbon dioxide (CO2), transitions to a gas upon recovery, simplifying post-processing steps, such as evaporation and drying procedures. Furthermore, CO2 is more cost-effective compared to organic solvents and can be sourced in high purity (>99.99%). On the other hand, CO2 experiences the Joule-Thomson cooling effect and undergoes an adiabatic expansion approximately 500 times to 1 when it is exposed to the lower atmospheric pressure at the outlet of the back pressure regulator. Consequently, during preparative separation, it is necessary to take measures to prevent contamination, such as condensed water from sample scattering and sudden temperature drops.
In this application note, it is demonstrated how the installation of the MCS-es, a gas-liquid separation nozzle, on the Z-axis arm of the Gilson 223 Sample Changer fraction collector facilitates the gradual collection of only the liquid phase. This innovative setup effectively minimizes scattering and condensation due to CO2 expansion, enabling efficient preparative separation with high recovery rates across a wide range of flow rates. In addition, it allows for the direct incorporation of make-up solvent into the MCS-es (patent pending). The achiral separation of a standard solution containing three components (1,000 µg/mL caffeine, 500 µg/mL anthracene, and 500 µg/mL fluorene in methanol) was performed using the LC-4000 Series Preparative SFC system in conjunction with the Gilson 223 Sample Chamber fraction collector equipped with the MCS–es.
Experimental
SFC System Configuration
| Liquid CO2 Pump | PU-4388 | Back Pressure Regulator (BPR) | BP-4340 |
| Modifier Pump | PU-4088* | Post-heater | HE-02 |
| Pre-heater | HE-01 | Heater Controller | HC-4068-01 |
| Heater Controller | HC-4068-01 | Make-up Pump | PU-4086* |
| Autosampler | AS-4358 | Fraction Valve | FV-4000-06 |
| Column Oven | CO-4060* | Fraction Collector | Gilson 223 Sample Chamber |
| Photo Diode Array (PDA) Detector | MD-4010* | Fume Hood | FH-4388 |
| *equipped with optional units | |||
SFC Conditions
| Column | YMC-Pack Pro C18 (30 mm I.D. x 250 mm L, 10 µm) |
| Mobile Phase (%) | Supercritical carbon dioxide (CO2)/Methanol (MeOH) (95/5) |
| Flow Rate | 120 mL/min |
| Column Temperature | 40 ºC |
| Pre-heat Temperature | 40 ºC |
| Wavelength | 200 – 650 nm; 215 nm (CH1), 270 nm (CH9), 258 nm (CH10), and 244 nm (CH11) |
| Back Pressure | 10 MPa |
| Post-heat Temperature | 60 ºC |
| Make-up Solvent | Methanol |
| Make-up Solvent Flow Rate | 10 mL/minute |
| Injection Volume | 1,000 µL |
| Standard Solution | 1,000 µg/mL caffeine, 500 µg/mL anthracene, and 500 µg/mL fluorene in methanol |
SFC Schematic Diagram
Results

1: Caffeine, 2: Anthracene, 3: Fluorene
Figure 1 shows the chromatogram of the standard solution containing three components (1,000 µg/mL caffeine, 500 µg/mL anthracene, and 500 µg/mL fluorene in methanol). Based on this chromatogram, a fraction collection method was created and used for the preparative separation of the standard solution.

1: Caffeine, 2: Anthracene, 3: Fluorene
Figure 2 shows the fraction collection results from the achiral preparative separation of the standard solution containing three components (caffeine, anthracene, and fluorene) based on time fractionation. In Figure 2, the green section represents the collection phase, the pink section represents the make-up phase, and the purple section represents the drop waiting time*1.
*1 The time taken to collect the modifier and make-up solvent remaining in the MCS-es after valve switching.

1: Caffeine (equivalent to 50 µg/mL),
2: Anthracene (equivalent to 25 µg/mL),
3: Fluorene (equivalent to 25 µg/mL),
*4: Solvent peak
Figure 3 shows the chromatograms obtained by re-measuring each fraction that was collected in Figure 2. Each fraction was diluted to 20 mL by co-washing with methanol so that caffeine, anthracene, and fluorene were each 50 µg/mL, 25 µg/mL, and 25 µg/mL, respectively (equivalent). These solutions were used for recovery rate measurements.
Table 1 shows the recovery rates for each component of the standard solution, calculated assuming the area values at the time of measurement of the standard solution as 100% (50 µg/mL caffeine, 25 µg/mL anthracene, and 25 µg/mL fluorene). Excellent recovery rates were obtained for all components.
Table 1. Recovery Rates of the Three Components (1: Caffeine, 2: Anthracene, 3: Fluorene) in the Standard Solution
| Caffeine | Anthracene | Fluorene | |
| Recovery Rate % | 98.06 | 94.82 | 93.57 |
Conclusion
The use of the Preparative SFC system in conjunction with the Gilson 223 Sample Chamber fraction collector equipped with MCS-es gas-liquid separation nozzle successfully achieved achiral separation and preparation of a standard solution containing 3 components (caffeine, anthracene, and fluorene) with excellent recovery rates.
The MCS-es can be used with a maximum total flow rate of up to 200 mL/min (under specified conditions). It also supports preparation at relatively low flow rates on an analytical scale, so it can be applied to preparation over a wide range of flow rates.
Keywords
Preparative Supercritical Fluid Chromatography, Preparative SFC, Prep Supercritical Fluid Chromatography, Prep SFC, supercritical carbon dioxide, supercritical CO2, gas-liquid separator, MCS-es, fraction collector, Gilson 223 Sample Changer, YMC-Pack Pro C18, achiral separation, achiral preparation

 Download This Application
Download This Application