Analysis of Automotive Fluids using FTIR with an ATR Accessory
August 24, 2022
Introduction
Liquids are among the most common types of samples submitted for analysis and comprises a vast array of pure compounds and mixed solutions. As an example, several liquids are used in an automobile in critical elements such as engine or drive train lubrication and to supply mundane functions like washing the windows or generating electrical power. This application note describes the analysis of several solutions used in automobiles.
The traditional infrared analysis method for fluid samples is to measure an infrared transmission spectrum of a thin film of the liquid contained between the windows of a liquid cell. Not all liquids, however, can be analyzed with infrared spectroscopy in this manner. For samples that are aqueous, viscous or chemically reactive, an IR liquid cell is cumbersome and labor intensive. Frequently, special windows with reduced spectral range must be used and the cell can be difficult to keep clean to prevent cross contamination.
Infrared analysis using Attenuated Total Reflectance (ATR) accessories like the diamond single-reflection ATR require no sample preparation and greatly simplify the collection of FTIR spectra. The liquid sample is placed onto the ATR crystal and the sample spectrum is collected. A volatiles cover supplied with the accessory can be used to cover the sample to prevent evaporation during analysis. The sample is then cleaned from the crystal surface and the accessory is ready to collect additional spectra. ATR analysis methods are less complicated than using liquid cells, are fast and require only a very small amount of sample.
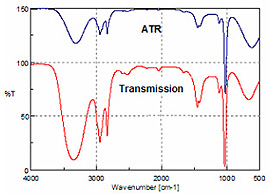
The resulting spectrum can be searched against a database of ATR spectra for positive identification. Despite changes in the relative peak intensity of the absorption bands, due to the physics of ATR accessories, spectra can also be compared to transmission data. As an example, Figure 1 shows a plot of the transmission and ATR spectra of methanol.
Experimental

Spectra were collected using an FTIR-460 FTIR spectrometer fitted with a diamond prism ATR accessory. Sample volumes of 20 uL were pipetted onto the ATR surface and spectra collected. No sample preparation was necessary to obtain the spectra and the sample can be easily wiped from the crystal surface after measurement. If necessary, a compatible solvent that will remove the sample is used to clean the diamond crystal.
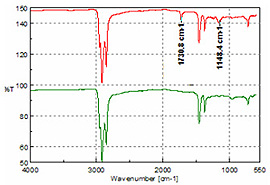
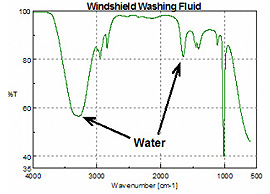
FTIR spectra, 64 scans at 4 cm-1 resolution were averaged to obtain the single-beam background and sample spectra. Figure 2 shows a spectrum of windshield washing solution and it can be seen that methanol (Figure 1) is the major component. Water increases the intensity and band broadening for the O-H stretch and bending modes.
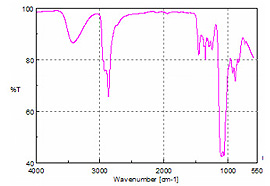
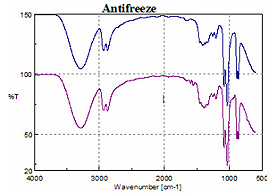
Figure 3 Shows the spectra of two samples of anti-freeze. While the spectra for the “normal” anti-freeze and the extended lifetime solution appeared identical, a subtraction of the standard ethylene glycol solution from the extended mileage formulation reveals a spectrum of the component(s) that presumably extends the anti-freeze lifetime.
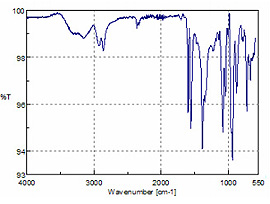
Analysis of 10W 30 engine oil, power steering fluid and two automatic transmission fluids (ATF 3 and ATF 4) all yielded spectra representative of long-chain aliphatics as illustrated in Figure 5. The only discernible difference is the additional peaks at 1730 and 1151 cm-1, which highlight a similar additive in the ATF and power steering samples.Figure 6 is a spectrum of brake fluid and was interpreted as a complex alcohol or possibly a diol, providing lubrication and compression characteristics.
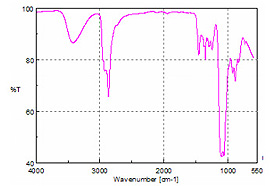
The most challenging sample was battery acid. Mainly sulfuric acid, battery acid is extremely corrosive and will etch or react with almost every standard infrared window or ATR element. By contrast, the diamond element of the ATR is not damaged during analysis. The spectrum of the acid solution is displayed as Figure 7.
Conclusion
The single reflection ATR is a simple, easy-to-use accessory for the analysis of liquids and solutions. The ATR technique is simple, rapid and very reliable for sample characterization. The analysis method is non-destructive and can be used to collect data from a minimal amount of sample.
Featured Products:

Analysis of Automotive Fluids using FTIR with an ATR Accessory
Introduction
Liquids are among the most common types of samples submitted for analysis and comprises a vast array of pure compounds and mixed solutions. As an example, several liquids are used in an automobile in critical elements such as engine or drive train lubrication and to supply mundane functions like washing the windows or generating electrical power. This application note describes the analysis of several solutions used in automobiles.

The traditional infrared analysis method for fluid samples is to measure an infrared transmission spectrum of a thin film of the liquid contained between the windows of a liquid cell. Not all liquids, however, can be analyzed with infrared spectroscopy in this manner. For samples that are aqueous, viscous or chemically reactive, an IR liquid cell is cumbersome and labor intensive. Frequently, special windows with reduced spectral range must be used and the cell can be difficult to keep clean to prevent cross contamination.

Infrared analysis using Attenuated Total Reflectance (ATR) accessories like the diamond single-reflection ATR require no sample preparation and greatly simplify the collection of FTIR spectra. The liquid sample is placed onto the ATR crystal and the sample spectrum is collected. A volatiles cover supplied with the accessory can be used to cover the sample to prevent evaporation during analysis. The sample is then cleaned from the crystal surface and the accessory is ready to collect additional spectra. ATR analysis methods are less complicated than using liquid cells, are fast and require only a very small amount of sample.

The resulting spectrum can be searched against a database of ATR spectra for positive identification. Despite changes in the relative peak intensity of the absorption bands, due to the physics of ATR accessories, spectra can also be compared to transmission data. As an example, Figure 1 shows a plot of the transmission and ATR spectra of methanol.

Experimental

Spectra were collected using an FTIR-460 FTIR spectrometer fitted with a diamond prism ATR accessory. Sample volumes of 20 uL were pipetted onto the ATR surface and spectra collected. No sample preparation was necessary to obtain the spectra and the sample can be easily wiped from the crystal surface after measurement. If necessary, a compatible solvent that will remove the sample is used to clean the diamond crystal.

FTIR spectra, 64 scans at 4 cm-1 resolution were averaged to obtain the single-beam background and sample spectra. Figure 2 shows a spectrum of windshield washing solution and it can be seen that methanol (Figure 1) is the major component. Water increases the intensity and band broadening for the O-H stretch and bending modes.

Figure 3 Shows the spectra of two samples of anti-freeze. While the spectra for the “normal” anti-freeze and the extended lifetime solution appeared identical, a subtraction of the standard ethylene glycol solution from the extended mileage formulation reveals a spectrum of the component(s) that presumably extends the anti-freeze lifetime.
Analysis of 10W 30 engine oil, power steering fluid and two automatic transmission fluids (ATF 3 and ATF 4) all yielded spectra representative of long-chain aliphatics as illustrated in Figure 5. The only discernible difference is the additional peaks at 1730 and 1151 cm-1, which highlight a similar additive in the ATF and power steering samples.Figure 6 is a spectrum of brake fluid and was interpreted as a complex alcohol or possibly a diol, providing lubrication and compression characteristics.
The most challenging sample was battery acid. Mainly sulfuric acid, battery acid is extremely corrosive and will etch or react with almost every standard infrared window or ATR element. By contrast, the diamond element of the ATR is not damaged during analysis. The spectrum of the acid solution is displayed as Figure 7.

Conclusion
The single reflection ATR is a simple, easy-to-use accessory for the analysis of liquids and solutions. The ATR technique is simple, rapid and very reliable for sample characterization. The analysis method is non-destructive and can be used to collect data from a minimal amount of sample.

 Download This Application
Download This Application
