Assessment of Higher Order Structure Similarities Between Innovators and Biosimilars using High-throughput Circular Dichroism Spectrometer
October 8, 2024
Introduction

The market for therapeutic antibodies has been dramatically expanding over the past decade. Antibody drugs exhibit therapeutic effects such as inhibiting the growth of malignant tumor cells and the activity of immune cells by binding antigens expressed in target cells with high affinity and specificity.
Such antibody drugs are expected to be effective therapeutic agents for as yet unmet medical needs. Many antibody drugs are being used worldwide, and along with leading antibody drugs called innovators, many biosimilars, which have the same amino acid sequence as the innovators but are produced using different expression cells, are also beginning to be used.
Biosimilars tend to be thought of as having the same function and structure as the innovators because they have the same amino acid sequence. However, antibodies are subjected to various stimuli and post-translational modifications during the production process, which may result in a significant loss of function. The main factors are thermal stimulation and fragmentation by proteases. Therefore, measuring changes in antibody structure due to stimulation or modification is an essential step in the research and development process, and for quality control of antibody drugs.
The ICH Q5E1) guideline for quality control of biological products states that the comparability of the higher order structure (HOS) of antibodies before and after a change in the manufacturing process should be objectively evaluated. Global guidance2, 3) from the FDA4) and EMA5) also states that HOS comparisons should be performed and secondary structure composition ratios for innovators and biosimilars should be evaluated. Circular dichroism (CD) spectroscopy is widely used to evaluate antibody quality, as it is a simple way to obtain information on the HOS of antibodies. To implement the above guidelines, there is a need for a method that can objectively and sensitively evaluate the similarity of CD spectra. The JASCO [qHOS] program, which was developed to meet these requirements, is capable of detecting significant differences in spectra with high sensitivity, and performing statistical tests.
Here we report the results of an HOS similarity assessment of RIABNITM (a biosimilar to MabThera/Rituxan®, the innovator of Rituximab), and Herceptin® (the innovator of Trastuzumab), using the JASCO HTCD Plus high-throughput CD spectrometer (Fig. 1) and the [qHOS] program.
Experimental
System
The HTCD Plus high-throughput circular dichroism spectrometer is capable of continuously measuring up to 192 samples (with two 96-well plates, up to 120 samples when using vials) in a fully automated manner, in addition to cleaning and drying samples, and is ideal for applications that require precise measurement of multiple samples, such as antibody drug similarity assessments.
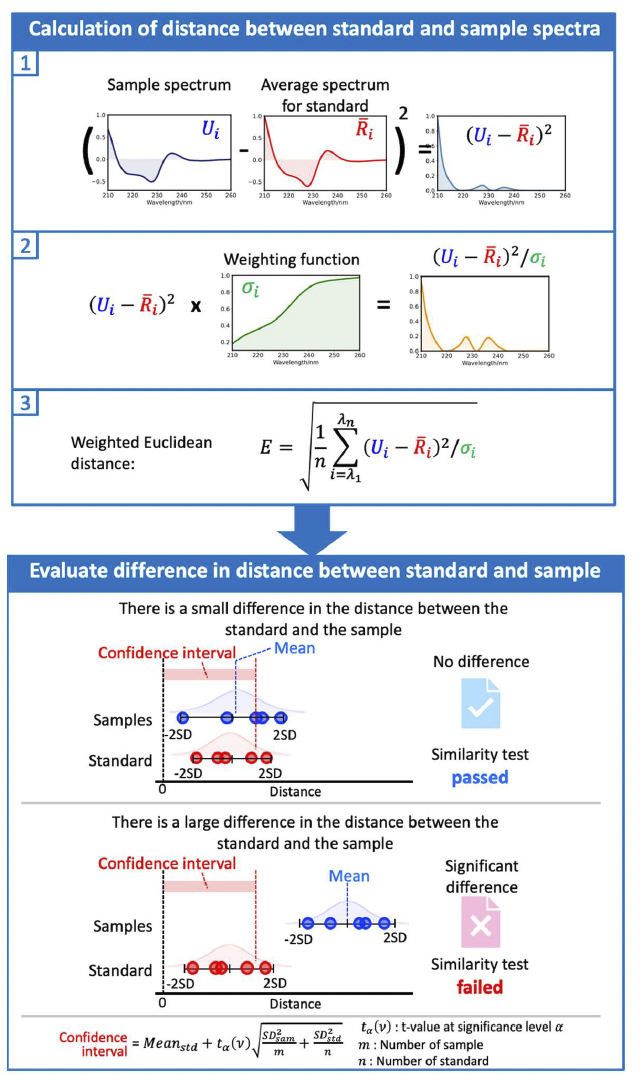
The [qHOS] program converts the spectral difference into a parameter called the “Euclidean distance”. By weighting the Euclidean distance to account for noise and spectral variation, differences between spectra can be detected with high sensitivity (“weighted Euclidean distance”). The [qHOS] program allows statistical significance testing to determine whether the differences between spectra are due to noise or sample preparation errors, or are in fact chemically meaningful differences (Fig. 2). Three types of statistical tests can be selected according to the purpose (Table 1).
| Table 1. Procedure for each statistical test | |
| Assay | Features |
| Student’s t-test | This method tests for significant differences in spectral distances, taking into account the variance in standard sample spectral distances. Data for multiple standard samples and a single unknown sample are used. This test is used for quality assessment of manufactured antibody drugs. |
| Welch’s t-test | This method tests for significant differences in spectral distances, taking into account the variances in both standard and sample spectral distances. Data for multiple standards and unknown samples are used. This test is used for comparing the HOS of innovators and biosimilars, the HOS for antibody drugs before and after manufacturing process changes, and for lot inspections. |
| Equivalence test (TOST) | This method tests for significant differences in spectral distances, taking into account the variances in both standard and sample spectral distances. In this method, it can be set a range (equivalence margin) in which the distance between the spectrum of each standard sample and the average spectrum of standard samples, and the distance between the spectrum of each unknown sample and the average spectrum of unknown samples, are equivalent respectively. Data for multiple standards and unknown samples are used. Tests can be performed based on the FDA guidance for similarity assessment.6) |
Samples
| Rituximab | Trastuzumab |
| MabThera (Innovator) RIABNI (Biosimilar) Both samples were prepared at a concentration of 10 mg/mL Additive: Sodium citrate dihydrate 7.4 mg/mL, Sodium chloride 9.0 mg/mL, Sodium hydroxide 9.0 mg/mL, Polysorbate 80 0.7 mg/mL | Herceptin (Innovator) Powder was dissolved in H2O to a concentration of 10 mg/mL Additives: Trehalose hydrate 4.7 mg/mL, L-histidine hydrochloride hydrate 0.11 mg/mL, L-histidine 7.4 x 10-2 mg/mL, Polysorbate 2.1 x 10-2 mg/mL |
Measurement Conditions
| Far-UV/CD spectrum | Near-UV/CD spectra/b> | |
| Bandwidth/b> | 1.0 nm | 1.0 nm |
| D.I.T./b> | 2 sec | 4 sec |
| Accumulations/b> | 4 times | 4 times |
| Sample Concentrations/b> | 0.5 mg/mL | 10 mg/mL |
| Scanning Speed/b> | 50 nm/min | 2 0 nm/min |
| Data Interval/b> | 0.1 nm | 0.1 nm |
| Optical Path Length/b> | 0.2 mm | 0.5 mm |
| Number of Samples/b> | 9 | 9 |
Analysis Conditions
| Distance Calculation Method | Euclidean distance |
| Concentration Correction | Absorbance (far-UV: Abs200, near-UV: 0.5 Abs280 ) |
| Smoothing | Savitzky-Golay |
| Weighting | Noise |
| Statistical Testing | Welch’s t-test |
| Significance Level | 0.05 |
Keywords
200-CD-0040, Antibody drugs, Higher order structure, HOS, Secondary structure, Tertiary structure, Biosimilars, Rituximab, MabThera®, RIABNITM, Trastuzumab, Herceptin®, Circular dichroism spectrometer, qHOS, Similarity assessment
Results
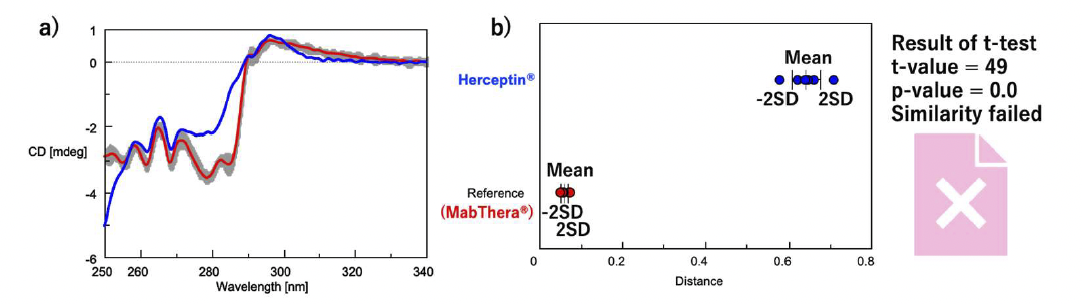
First, to confirm that this system can discriminate between the tertiary structures of antibody drugs with different targets and formulation conditions, we determined the similarity of the tertiary structures of MabThera, the innovator of Rituximab, and Herceptin, the innovator of Trastuzumab (Fig. 3). The shapes of the near-UV/CD spectra of MabThera and Herceptin differ significantly (Fig. 3a). Similarly, the average distances between MabThera and Herceptin calculated from the CD spectra show a large difference (Fig. 3b). The p-value obtained from the t-test is below the significance level of 0.05, indicating that Herceptin has a different tertiary structure to MabThera. These results show that the system can clearly distinguish differences in the tertiary structure of different antibody drugs.
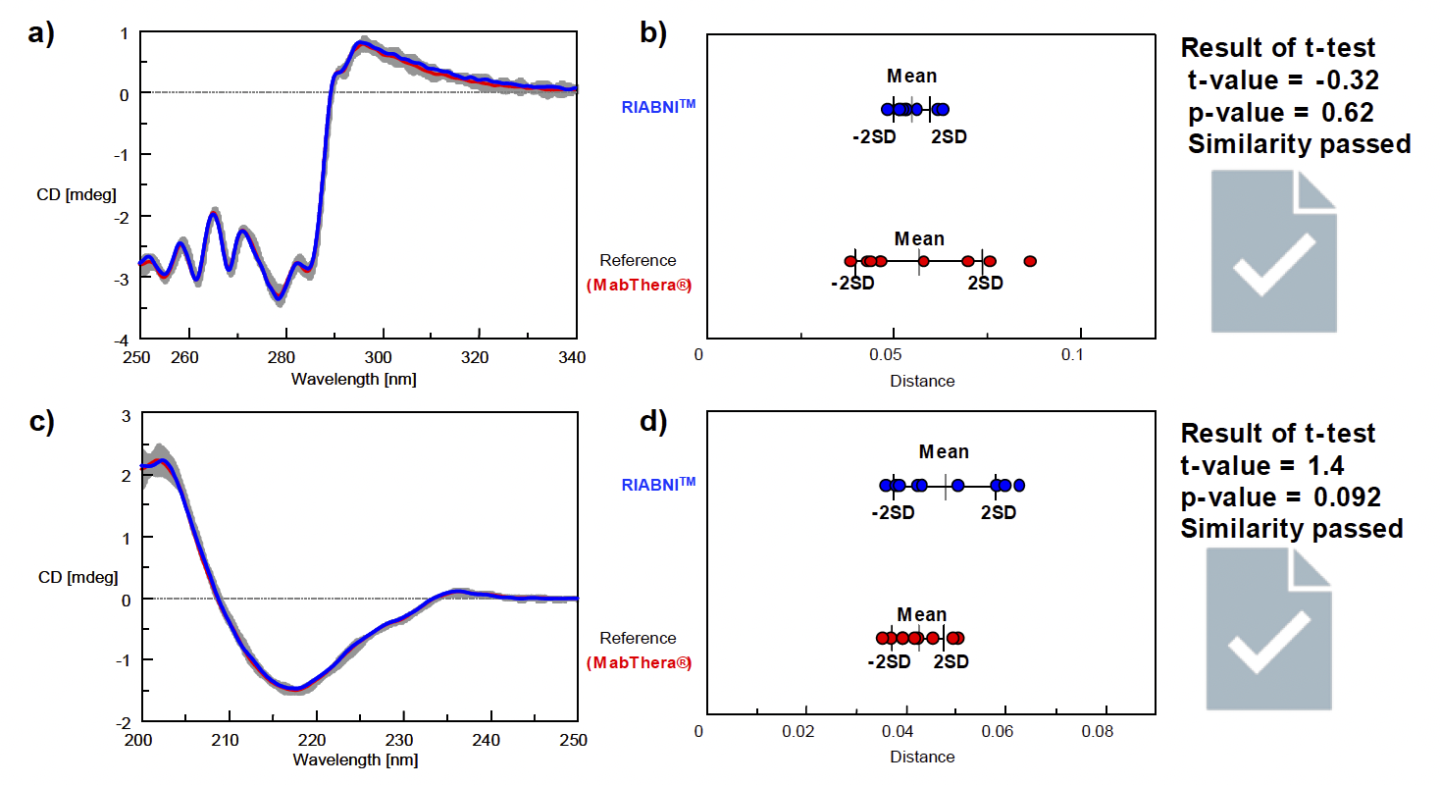
Next, we determined the similarity of the tertiary and secondary structures of RIABNI and MabThera (Fig. 4). The results for the tertiary structure similarity obtained from near-UV/CD spectra are shown in Figures 4a and 4b, and the results for the secondary structure similarity obtained from far-UV/CD spectra are shown in Figures 4c and 4d.
For both the near- and far-UV results, the CD spectrum of the biosimilar RIABNI is in excellent agreement with that of the innovator MabThera, and the calculated distance is very small. The results of the t-test showed that the p-value was larger than the significance level of 0.05, objectively confirming that RIABNI has the same tertiary and secondary structures as MabThera.
Conclusion
Using the HTCD Plus high-throughput CD spectrometer and the [qHOS] program, we found significant differences between the tertiary structures of MabThera and Herceptin, two antibody drugs with different targets and formulation conditions. On the other hand, RIABNI, a biosimilar of MabThera, was determined to have the same tertiary and secondary structures as MabThera based on statistical testing. Using this system, it is possible to not only determine the similarity between the HOS of a biosimilar and an innovator, but also to accurately and easily evaluate whether the HOS of the antibody drug is changed by post-translational modification or external stress.
Required Products and Software
System Configuration
| Model | Description | Part Number | |
| Main Unit | J-1500-450ST | Spectrometer *1), *2) | 7000-J006A |
| Options | FLM-525 | N2 gas flow meter | 7069-J025A |
| HTCD Plus (Microplate rack type) *3) | |||
| 0.2 mm flow cell | 7069-J186A | ||
| 0.5 mm flow cell | 7069-J415A | ||
| Program | JWQHOS-531 | qHOS Higher order structure similarity evaluation software for Spectra Manager 2/2.5 | 4880-J111A |
*1) J-1500-150ST (P/N: 7000-J005A) also can be used instead of J-1500-450ST. In this case, a water circulator for cooling the light source is not necessary.
*2) A water circulator for cooling the light source, a nitrogen gas cylinder, and a regulator for the cylinder are required separately.
*3) In addition to the microplate rack type, the vial rack type is also available. The configuration and consumables are shown in the tables below. The consumables are included in the standard configuration.
| HTCD Plus Configuration | ||
| Items | Microplate Rack Type | Vial Rack Type |
| ASU-800CD Autosampler | ||
| SRA-841 Microplate rack | ||
| SRA-842 Vial rack | ||
| ADU-835 Drying pump unit | ||
| HTCD Plus System case | ||
| JFLC-515 Peltier Thermostatted Flow Cell Holder | ||
| ASP-849 Syringe pump for ASU-800CD | ||
| 1 mm flow cell | ||
| MP weight |
| HTCD Plus Consumables | ||
| Items | Part Number | Vial Rack Type |
| Teflon tube cutter | 0507-7426 | |
| 96-well microplate, 200 µl, 100 pcs./set, BRG-96S-100 | 7069-H815A | |
| 9-SCK(B)-ST1X Screw cap with pre-cut seal, blue, 500 pcs./set | 0402-0084 | Vial rack type |
| 03-FISV(A) Screw thread vial, 0.3 ml, Amber | 0410-0173 | Vial rack type |
| Angled cut needle for ASU-800 | 6989-H130A |
References
1) International Conference on Harmonisation (ICH) of Technical Requirements for Registration of Pharmaceuticals for Human Use. ICH AUTHORIZED MANUFACTURING CHANGES FOR THERAPEUTIC MABS 833 Harmonised Tripartite Guideline Comparability of Biotechnological/Biological Products Subject to Changes in their Manufacturing Process Q5E. 2004
2) Department of Biotechnology, Government of India. Guidelines on similar biologics: Regulatory requirements for marketing authorization in India. 2012
3) Center for Drug Evaluation of China National Medical Product Administration. Technical guidelines for development and evaluation of biosimilars. 2015
4) US Food and Drug Administration. Development of Therapeutic Protein Biosimilars: Comparative Analytical Assessment and Other Quality-Related Considerations. 2019
5) European Medicines Agency. Guideline on similar biological medicinal products containing biotechnology-derived proteins as active substance: quality issues (revision 1). 2014
6) Y. Tsong, X. Dong, and M. Shen, Development of statistical methods for analytical similarity assessment, J. Biopharm. Stat. 2017
Related Products
-
Automated HTCD
-

Highest performance with a wide range of accessories for maximum flexibility to meet complex research demands.
J-1500
-
Spectra Manager™

Assessment of Higher Order Structure Similarities Between Innovators and Biosimilars using High-throughput Circular Dichroism Spectrometer
Introduction

The market for therapeutic antibodies has been dramatically expanding over the past decade. Antibody drugs exhibit therapeutic effects such as inhibiting the growth of malignant tumor cells and the activity of immune cells by binding antigens expressed in target cells with high affinity and specificity.
Such antibody drugs are expected to be effective therapeutic agents for as yet unmet medical needs. Many antibody drugs are being used worldwide, and along with leading antibody drugs called innovators, many biosimilars, which have the same amino acid sequence as the innovators but are produced using different expression cells, are also beginning to be used.
Biosimilars tend to be thought of as having the same function and structure as the innovators because they have the same amino acid sequence. However, antibodies are subjected to various stimuli and post-translational modifications during the production process, which may result in a significant loss of function. The main factors are thermal stimulation and fragmentation by proteases. Therefore, measuring changes in antibody structure due to stimulation or modification is an essential step in the research and development process, and for quality control of antibody drugs.
The ICH Q5E1) guideline for quality control of biological products states that the comparability of the higher order structure (HOS) of antibodies before and after a change in the manufacturing process should be objectively evaluated. Global guidance2, 3) from the FDA4) and EMA5) also states that HOS comparisons should be performed and secondary structure composition ratios for innovators and biosimilars should be evaluated. Circular dichroism (CD) spectroscopy is widely used to evaluate antibody quality, as it is a simple way to obtain information on the HOS of antibodies. To implement the above guidelines, there is a need for a method that can objectively and sensitively evaluate the similarity of CD spectra. The JASCO [qHOS] program, which was developed to meet these requirements, is capable of detecting significant differences in spectra with high sensitivity, and performing statistical tests.
Here we report the results of an HOS similarity assessment of RIABNITM (a biosimilar to MabThera/Rituxan®, the innovator of Rituximab), and Herceptin® (the innovator of Trastuzumab), using the JASCO HTCD Plus high-throughput CD spectrometer (Fig. 1) and the [qHOS] program.
Experimental
System
The HTCD Plus high-throughput circular dichroism spectrometer is capable of continuously measuring up to 192 samples (with two 96-well plates, up to 120 samples when using vials) in a fully automated manner, in addition to cleaning and drying samples, and is ideal for applications that require precise measurement of multiple samples, such as antibody drug similarity assessments.
The [qHOS] program converts the spectral difference into a parameter called the “Euclidean distance”. By weighting the Euclidean distance to account for noise and spectral variation, differences between spectra can be detected with high sensitivity (“weighted Euclidean distance”). The [qHOS] program allows statistical significance testing to determine whether the differences between spectra are due to noise or sample preparation errors, or are in fact chemically meaningful differences (Fig. 2). Three types of statistical tests can be selected according to the purpose (Table 1).
| Table 1. Procedure for each statistical test | |
| Assay | Features |
| Student’s t-test | This method tests for significant differences in spectral distances, taking into account the variance in standard sample spectral distances. Data for multiple standard samples and a single unknown sample are used. This test is used for quality assessment of manufactured antibody drugs. |
| Welch’s t-test | This method tests for significant differences in spectral distances, taking into account the variances in both standard and sample spectral distances. Data for multiple standards and unknown samples are used. This test is used for comparing the HOS of innovators and biosimilars, the HOS for antibody drugs before and after manufacturing process changes, and for lot inspections. |
| Equivalence test (TOST) | This method tests for significant differences in spectral distances, taking into account the variances in both standard and sample spectral distances. In this method, it can be set a range (equivalence margin) in which the distance between the spectrum of each standard sample and the average spectrum of standard samples, and the distance between the spectrum of each unknown sample and the average spectrum of unknown samples, are equivalent respectively. Data for multiple standards and unknown samples are used. Tests can be performed based on the FDA guidance for similarity assessment.6) |
Samples
| Rituximab | Trastuzumab |
| MabThera (Innovator) RIABNI (Biosimilar) Both samples were prepared at a concentration of 10 mg/mL Additive: Sodium citrate dihydrate 7.4 mg/mL, Sodium chloride 9.0 mg/mL, Sodium hydroxide 9.0 mg/mL, Polysorbate 80 0.7 mg/mL | Herceptin (Innovator) Powder was dissolved in H2O to a concentration of 10 mg/mL Additives: Trehalose hydrate 4.7 mg/mL, L-histidine hydrochloride hydrate 0.11 mg/mL, L-histidine 7.4 x 10-2 mg/mL, Polysorbate 2.1 x 10-2 mg/mL |

Measurement Conditions
| Far-UV/CD spectrum | Near-UV/CD spectra/b> | |
| Bandwidth/b> | 1.0 nm | 1.0 nm |
| D.I.T./b> | 2 sec | 4 sec |
| Accumulations/b> | 4 times | 4 times |
| Sample Concentrations/b> | 0.5 mg/mL | 10 mg/mL |
| Scanning Speed/b> | 50 nm/min | 2 0 nm/min |
| Data Interval/b> | 0.1 nm | 0.1 nm |
| Optical Path Length/b> | 0.2 mm | 0.5 mm |
| Number of Samples/b> | 9 | 9 |
Analysis Conditions
| Distance Calculation Method | Euclidean distance |
| Concentration Correction | Absorbance (far-UV: Abs200, near-UV: 0.5 Abs280 ) |
| Smoothing | Savitzky-Golay |
| Weighting | Noise |
| Statistical Testing | Welch’s t-test |
| Significance Level | 0.05 |
Results
First, to confirm that this system can discriminate between the tertiary structures of antibody drugs with different targets and formulation conditions, we determined the similarity of the tertiary structures of MabThera, the innovator of Rituximab, and Herceptin, the innovator of Trastuzumab (Fig. 3). The shapes of the near-UV/CD spectra of MabThera and Herceptin differ significantly (Fig. 3a). Similarly, the average distances between MabThera and Herceptin calculated from the CD spectra show a large difference (Fig. 3b). The p-value obtained from the t-test is below the significance level of 0.05, indicating that Herceptin has a different tertiary structure to MabThera. These results show that the system can clearly distinguish differences in the tertiary structure of different antibody drugs.

Next, we determined the similarity of the tertiary and secondary structures of RIABNI and MabThera (Fig. 4). The results for the tertiary structure similarity obtained from near-UV/CD spectra are shown in Figures 4a and 4b, and the results for the secondary structure similarity obtained from far-UV/CD spectra are shown in Figures 4c and 4d.
For both the near- and far-UV results, the CD spectrum of the biosimilar RIABNI is in excellent agreement with that of the innovator MabThera, and the calculated distance is very small. The results of the t-test showed that the p-value was larger than the significance level of 0.05, objectively confirming that RIABNI has the same tertiary and secondary structures as MabThera.

Conclusion
Using the HTCD Plus high-throughput CD spectrometer and the [qHOS] program, we found significant differences between the tertiary structures of MabThera and Herceptin, two antibody drugs with different targets and formulation conditions. On the other hand, RIABNI, a biosimilar of MabThera, was determined to have the same tertiary and secondary structures as MabThera based on statistical testing. Using this system, it is possible to not only determine the similarity between the HOS of a biosimilar and an innovator, but also to accurately and easily evaluate whether the HOS of the antibody drug is changed by post-translational modification or external stress.
Keywords
200-CD-0040, Antibody drugs, Higher order structure, HOS, Secondary structure, Tertiary structure, Biosimilars, Rituximab, MabThera®, RIABNITM, Trastuzumab, Herceptin®, Circular dichroism spectrometer, qHOS, Similarity assessment
Required Products and Software
System Configuration
| Model | Description | Part Number | |
| Main Unit | J-1500-450ST | Spectrometer *1), *2) | 7000-J006A |
| Options | FLM-525 | N2 gas flow meter | 7069-J025A |
| HTCD Plus (Microplate rack type) *3) | |||
| 0.2 mm flow cell | 7069-J186A | ||
| 0.5 mm flow cell | 7069-J415A | ||
| Program | JWQHOS-531 | qHOS Higher order structure similarity evaluation software for Spectra Manager 2/2.5 | 4880-J111A |
*1) J-1500-150ST (P/N: 7000-J005A) also can be used instead of J-1500-450ST. In this case, a water circulator for cooling the light source is not necessary.
*2) A water circulator for cooling the light source, a nitrogen gas cylinder, and a regulator for the cylinder are required separately.
*3) In addition to the microplate rack type, the vial rack type is also available. The configuration and consumables are shown in the tables below. The consumables are included in the standard configuration.
| HTCD Plus Configuration | ||
| Items | Microplate Rack Type | Vial Rack Type |
| ASU-800CD Autosampler | ||
| SRA-841 Microplate rack | ||
| SRA-842 Vial rack | ||
| ADU-835 Drying pump unit | ||
| HTCD Plus System case | ||
| JFLC-515 Peltier Thermostatted Flow Cell Holder | ||
| ASP-849 Syringe pump for ASU-800CD | ||
| 1 mm flow cell | ||
| MP weight |
| HTCD Plus Consumables | ||
| Items | Part Number | Vial Rack Type |
| Teflon tube cutter | 0507-7426 | |
| 96-well microplate, 200 µl, 100 pcs./set, BRG-96S-100 | 7069-H815A | |
| 9-SCK(B)-ST1X Screw cap with pre-cut seal, blue, 500 pcs./set | 0402-0084 | Vial rack type |
| 03-FISV(A) Screw thread vial, 0.3 ml, Amber | 0410-0173 | Vial rack type |
| Angled cut needle for ASU-800 | 6989-H130A |
References
1) International Conference on Harmonisation (ICH) of Technical Requirements for Registration of Pharmaceuticals for Human Use. ICH AUTHORIZED MANUFACTURING CHANGES FOR THERAPEUTIC MABS 833 Harmonised Tripartite Guideline Comparability of Biotechnological/Biological Products Subject to Changes in their Manufacturing Process Q5E. 2004
2) Department of Biotechnology, Government of India. Guidelines on similar biologics: Regulatory requirements for marketing authorization in India. 2012
3) Center for Drug Evaluation of China National Medical Product Administration. Technical guidelines for development and evaluation of biosimilars. 2015
4) US Food and Drug Administration. Development of Therapeutic Protein Biosimilars: Comparative Analytical Assessment and Other Quality-Related Considerations. 2019
5) European Medicines Agency. Guideline on similar biological medicinal products containing biotechnology-derived proteins as active substance: quality issues (revision 1). 2014
6) Y. Tsong, X. Dong, and M. Shen, Development of statistical methods for analytical similarity assessment, J. Biopharm. Stat. 2017

 Download This Application
Download This Application
