Circular Dichroism Spectrometry for the Analysis of Amphetamines
September 8, 2023
Introduction
Amphetamines are controlled substances which are potent stimulants that induce feelings of strength and energy while decreasing the need to sleep or eat. The majority of illegal amphetamine products are racemic; consequently, both isomers will appear in the biological fluids unchanged. In comparison the break-down products formed by the metabolism of most pharmaceutical drugs show the appearance of pure amphetamine enantiomers. Circular dichroism is a form of light absorption spectroscopy that measures the difference in absorbance of right- and leftcircularly polarized light (rather than the commonly used absorbance of isotropic light) by a substance. Since different enantiomers absorb different portions of the light, it provides and excellent means for enantiomer detection. In this application CD will be used to study the isomers of amphetamine and methamphetamine.
Amphetamines are synthetic drugs that stimulate the central nervous system. They are structurally similar to ephedrine, a natural stimulant found in plants and to the hormone adrenaline. They are available legally by prescription and were once widely prescribed as a cureball for everything from asthma to epilepsy. Today, medical use of amphetamines is limited to treating minimal brain dysfunction (MBD) in children and narcolepsy, a rare disorder in which an individual suddenly falls into an uncontrollable deep sleep.
Amphetamines are a popular “street” drug. Legally produced amphetamines are sold illegally on the black market with variations in quality and quantity. Enterprising chemists have also developed a “lookalike” amphetamine manufactured to look like real amphetamines and mimic their effects. These are commonly referred to as “speed” or “uppers”. These drugs contain varying amounts of less potent stimulants such as caffeine, ephedrine and phenylpropanolamine-all legal substances that are usually found in over-the-counter diet pills and decongestants.
The majority of illegal amphetamine products are racemic; consequently, both isomers will appear in the biological fluids unchanged. In comparison the break-down products formed by the metabolism of most pharmaceutical drugs show the appearance of pure amphetamine enantiomers. A large portion of abused amphetamines are produced surreptitiously by various methods. This produces products with different qualities depending on the type of synthesis as well as on the chemicals used. The identification of by-products of the synthesis and the determination of the enantiomeric composition of the active substance as well as that of the impurities being present in the preparations, are important aims of the forensic and pharmacological analysis. It is also useful for both comparative purposes and for the evaluation of the production methods. In this note we will show the applicability of CD to the determination of enantiomeric composition.
Experimental

The individual isomers of amphetamine and methamphetamine (1mg/mL in methanol) were purchased from Altech and used without further preparation. CD spectra were collected at room temperature using a Jasco J-1500 CD Spectrometer in a low volume 1cm path length cell. The response time used was 1sec with a data pitch of 0.1nm and a scan speed of 100nm/min, giving complete analysis in less than 2 minutes.
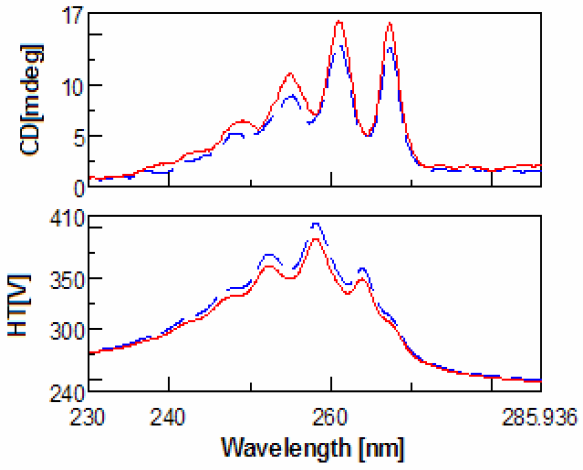
(red).
Results
Evaluation of the CD spectra of methamphetamine and amphetamine show very few differences. This is due to the fact that meth-amphetamine is composed of an amphetamine molecule with an additional methyl group attached to its nitrogen (amine group). Figures 1-2 show the scans of the isomers of amphetamine and meth-amphetamine.
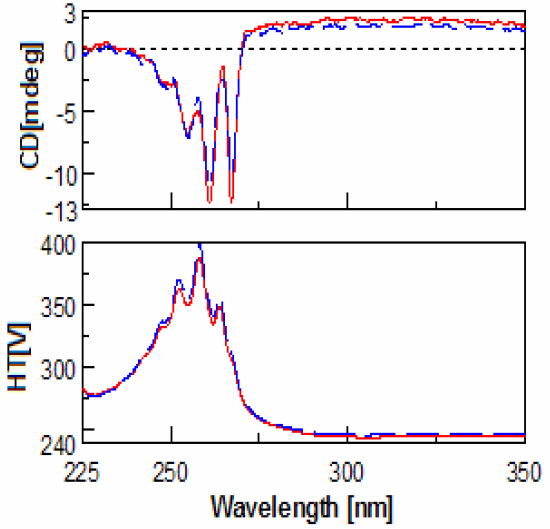
(red).
The most important use for CD is chiral detection and determination, and in the case methamphetamine is very useful for identification of the enantiomers. (Figure 3) Another important feature of the data is the HT[V] which is easily converted via software into absorbance units. Thus UV data is collected for every sample at the same time. Since legal amphetamines are detected as pure enantiomers and street versions racemic, CD is a quick method for determining prescription from illegal versions. CD is also an effective method for determining which enantiomer is used in the drug as an aid to identification.

Featured Products:
-

Highest performance with a wide range of accessories for maximum flexibility to meet complex research demands.
J-1500
-
J-1700
-
Automated HTCD

Circular Dichroism Spectrometry for the Analysis of Amphetamines
Introduction
Amphetamines are controlled substances which are potent stimulants that induce feelings of strength and energy while decreasing the need to sleep or eat. The majority of illegal amphetamine products are racemic; consequently, both isomers will appear in the biological fluids unchanged. In comparison the break-down products formed by the metabolism of most pharmaceutical drugs show the appearance of pure amphetamine enantiomers. Circular dichroism is a form of light absorption spectroscopy that measures the difference in absorbance of right- and leftcircularly polarized light (rather than the commonly used absorbance of isotropic light) by a substance. Since different enantiomers absorb different portions of the light, it provides and excellent means for enantiomer detection. In this application CD will be used to study the isomers of amphetamine and methamphetamine.
Amphetamines are synthetic drugs that stimulate the central nervous system. They are structurally similar to ephedrine, a natural stimulant found in plants and to the hormone adrenaline. They are available legally by prescription and were once widely prescribed as a cureball for everything from asthma to epilepsy. Today, medical use of amphetamines is limited to treating minimal brain dysfunction (MBD) in children and narcolepsy, a rare disorder in which an individual suddenly falls into an uncontrollable deep sleep.
Amphetamines are a popular “street” drug. Legally produced amphetamines are sold illegally on the black market with variations in quality and quantity. Enterprising chemists have also developed a “lookalike” amphetamine manufactured to look like real amphetamines and mimic their effects. These are commonly referred to as “speed” or “uppers”. These drugs contain varying amounts of less potent stimulants such as caffeine, ephedrine and phenylpropanolamine-all legal substances that are usually found in over-the-counter diet pills and decongestants.
The majority of illegal amphetamine products are racemic; consequently, both isomers will appear in the biological fluids unchanged. In comparison the break-down products formed by the metabolism of most pharmaceutical drugs show the appearance of pure amphetamine enantiomers. A large portion of abused amphetamines are produced surreptitiously by various methods. This produces products with different qualities depending on the type of synthesis as well as on the chemicals used. The identification of by-products of the synthesis and the determination of the enantiomeric composition of the active substance as well as that of the impurities being present in the preparations, are important aims of the forensic and pharmacological analysis. It is also useful for both comparative purposes and for the evaluation of the production methods. In this note we will show the applicability of CD to the determination of enantiomeric composition.
Experimental

The individual isomers of amphetamine and methamphetamine (1mg/mL in methanol) were purchased from Altech and used without further preparation. CD spectra were collected at room temperature using a Jasco J-1500 CD Spectrometer in a low volume 1cm path length cell. The response time used was 1sec with a data pitch of 0.1nm and a scan speed of 100nm/min, giving complete analysis in less than 2 minutes.

(red).
Results
Evaluation of the CD spectra of methamphetamine and amphetamine show very few differences. This is due to the fact that meth-amphetamine is composed of an amphetamine molecule with an additional methyl group attached to its nitrogen (amine group). Figures 1-2 show the scans of the isomers of amphetamine and meth-amphetamine.

(red).
The most important use for CD is chiral detection and determination, and in the case methamphetamine is very useful for identification of the enantiomers. (Figure 3) Another important feature of the data is the HT[V] which is easily converted via software into absorbance units. Thus UV data is collected for every sample at the same time. Since legal amphetamines are detected as pure enantiomers and street versions racemic, CD is a quick method for determining prescription from illegal versions. CD is also an effective method for determining which enantiomer is used in the drug as an aid to identification.


 Download This Application
Download This Application
