Detection of Chiral Drugs Using HPLC with Circular Dichroism Detection
August 11, 2022
Introduction
Recent American Food and Drug Administration guidelines have effectively determined that mixtures of chiral compounds can no longer be brought to the pharmaceuticals market place. For new pharmaceuticals, each chiral form must be pure and separately assessed for registration. The need for chiral detection in the development of pharmaceuticals is increasing due to regulatory changes and the demand for single isomers. The key pharmaceutical industry objective is now ‘racemic switching’ and single-isomer drugs. These guidelines require a means for chiral detection and compound separation.
Different chiral forms of molecules can have profoundly different biological effects. For example, one chiral form of the drug naproxen has 28 times the anti-inflammatory activity of the opposite chiral relative. Another example is the synthetic form of dopamine used to treat Parkinson’s disease: one isomer acts on the nerve cells to control the patient’s tremors whilst the other form is actually toxic to nerve cells. In crop protection it has been found that one chiral form of the pesticide Permethrine is much more toxic to insects than the other.
Currently there is little control over which chiral form of a chemical compound is formed during a production process. This lack of control generally results in production of equal amounts of both chiral forms. At the molecular level the two chiral partners have identical physical properties and therefore are very difficult and expensive to separate. A far superior alternative to separation would be processes in which single pure chiral compounds are formed in the first place. This application note will describe chiral detection of several over the counter medications using HPLC.
Experimental

A prescription Naproxen tablet (500mg) and an over the counter naproxen sodium tablet (220mg), were separately ground. The tablets were dissolved in ethanol and sonicated for 15 minutes. These solutions were then diluted to a final concentration of 5µg/µL and filtered through a 0.45µm PVDF syringe filter. A Jasco HPLC system with a CD, OR, and fluorescence detector was used. Injections size was 1µL. Separations were accomplished using a ChiralPak AD column (4.6 x 250mm) with a 100% EtOH with (0.1% TFA) mobile phase and a flow rate of 1.0 mL/min. CD/UV were monitored at 260nm for the naproxen and 310nm for the Coumadin.
Prescription Nexium and Prilosec were also examined by grinding the tablets and dissolving them in ethanol, and sonicating for 20 minutes. These solutions were brought to a final concentration 0f 1µg/µL with an injection size of 2µL. Separations were accomplished using a ChiralPak AD column (4.6 x 250mm) with a 100% EtOH mobile phase and a flow rate of 0.9mL/min.
Results
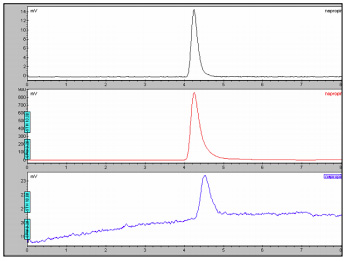
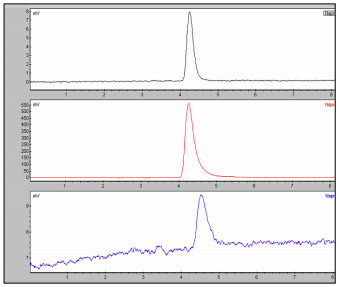
The chromatograms of Naproxen and the naproxen sodium are shown in Figures 1-2. Naproxen is used to relieve the pain, and inflammation caused by arthritis, and other inflammatory conditions. One chiral form of the drug naproxen (S-enantiomer) has 28 times the antiinflammatory activity of the opposite chiral relative. The (R) isomer is reported to be a liver toxin. Both the Naproxen and the sodium salt showed only one peak in the CD chromatogram as expected. This indicates that both the prescription and the over the counter versions have are meeting the requirements to be enantiomerically pure.
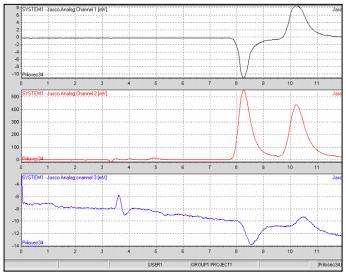
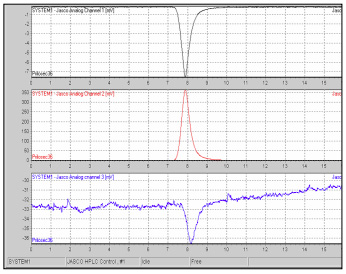
The chromatograms for the Nexium and the Prilosec are shown in Figures 3-4. Prilosec is a proton pump inhibitor used to treat ulcers, heartburn, acid reflux, or Zollinger-Ellison syndrome Nexium, (esomeprazole), is the S enantiomer of omeprazole, the active ingredient in Prilosec. Nexium was one of the first pharmaceuticals to be repatented and marketed as a pure enantiomer of a formerly mixed enantiomer drug. The Nexium has a superior clinical efficacy due to its higher and more consistent bioavailability. As expected, the CD of the prilosec shows the inclusion of both enantiomers while in the Nexium only the esomeprazole enantiomer is detected.
Conclusion
HPLC with CD detection is an excellent method for the separation and detection of chiral compounds including pharmaceuticals. Both the fluorescence and the CD detectors were very sensitive with respect to concentration; however the fluorescence detector can not give any indication as to which enantiomer is which. The optical rotation detector is capable of discriminating the enantiomers, but in all the materials studied was found to be much less sensitive. In general, the best materials for use with the OR are sugars. Jasco Inc. currently offers the world’s only Circular Dichroism detector for HPLC.
Featured Products:

Detection of Chiral Drugs Using HPLC with Circular Dichroism Detection
Introduction
Recent American Food and Drug Administration guidelines have effectively determined that mixtures of chiral compounds can no longer be brought to the pharmaceuticals market place. For new pharmaceuticals, each chiral form must be pure and separately assessed for registration. The need for chiral detection in the development of pharmaceuticals is increasing due to regulatory changes and the demand for single isomers. The key pharmaceutical industry objective is now ‘racemic switching’ and single-isomer drugs. These guidelines require a means for chiral detection and compound separation.
Different chiral forms of molecules can have profoundly different biological effects. For example, one chiral form of the drug naproxen has 28 times the anti-inflammatory activity of the opposite chiral relative. Another example is the synthetic form of dopamine used to treat Parkinson’s disease: one isomer acts on the nerve cells to control the patient’s tremors whilst the other form is actually toxic to nerve cells. In crop protection it has been found that one chiral form of the pesticide Permethrine is much more toxic to insects than the other.
Currently there is little control over which chiral form of a chemical compound is formed during a production process. This lack of control generally results in production of equal amounts of both chiral forms. At the molecular level the two chiral partners have identical physical properties and therefore are very difficult and expensive to separate. A far superior alternative to separation would be processes in which single pure chiral compounds are formed in the first place. This application note will describe chiral detection of several over the counter medications using HPLC.
Experimental

A prescription Naproxen tablet (500mg) and an over the counter naproxen sodium tablet (220mg), were separately ground. The tablets were dissolved in ethanol and sonicated for 15 minutes. These solutions were then diluted to a final concentration of 5µg/µL and filtered through a 0.45µm PVDF syringe filter. A Jasco HPLC system with a CD, OR, and fluorescence detector was used. Injections size was 1µL. Separations were accomplished using a ChiralPak AD column (4.6 x 250mm) with a 100% EtOH with (0.1% TFA) mobile phase and a flow rate of 1.0 mL/min. CD/UV were monitored at 260nm for the naproxen and 310nm for the Coumadin.
Prescription Nexium and Prilosec were also examined by grinding the tablets and dissolving them in ethanol, and sonicating for 20 minutes. These solutions were brought to a final concentration 0f 1µg/µL with an injection size of 2µL. Separations were accomplished using a ChiralPak AD column (4.6 x 250mm) with a 100% EtOH mobile phase and a flow rate of 0.9mL/min.
Results

The chromatograms of Naproxen and the naproxen sodium are shown in Figures 1-2. Naproxen is used to relieve the pain, and inflammation caused by arthritis, and other inflammatory conditions. One chiral form of the drug naproxen (S-enantiomer) has 28 times the antiinflammatory activity of the opposite chiral relative. The (R) isomer is reported to be a liver toxin. Both the Naproxen and the sodium salt showed only one peak in the CD chromatogram as expected. This indicates that both the prescription and the over the counter versions have are meeting the requirements to be enantiomerically pure.


The chromatograms for the Nexium and the Prilosec are shown in Figures 3-4. Prilosec is a proton pump inhibitor used to treat ulcers, heartburn, acid reflux, or Zollinger-Ellison syndrome Nexium, (esomeprazole), is the S enantiomer of omeprazole, the active ingredient in Prilosec. Nexium was one of the first pharmaceuticals to be repatented and marketed as a pure enantiomer of a formerly mixed enantiomer drug. The Nexium has a superior clinical efficacy due to its higher and more consistent bioavailability. As expected, the CD of the prilosec shows the inclusion of both enantiomers while in the Nexium only the esomeprazole enantiomer is detected.

Conclusion
HPLC with CD detection is an excellent method for the separation and detection of chiral compounds including pharmaceuticals. Both the fluorescence and the CD detectors were very sensitive with respect to concentration; however the fluorescence detector can not give any indication as to which enantiomer is which. The optical rotation detector is capable of discriminating the enantiomers, but in all the materials studied was found to be much less sensitive. In general, the best materials for use with the OR are sugars. Jasco Inc. currently offers the world’s only Circular Dichroism detector for HPLC.

 Download This Application
Download This Application
