High Speed Analysis of Dabsyl Amino Acids in Collagen using UHPLC
January 5, 2024
Introduction
Collagen, one of the proteins that has been recently attracting attention from the viewpoint of health and beauty, is a main constituent of corium which gives skin vitality and keeps the skin soft without wrinkles. The amino acid structure of collagen is an arrangement of glycine sequencing at every 3rd residue and contains other amino acids such as proline, hydroxyproline and alanine. In addition hydroxyproline and hydroxylysine are present in collagen. In order to measure the constituent amino acids of proteins or peptides such as collagen, sample preparation such as hydrolysis is required. A DAB-Label kit is available from JASCO for amino acid derivatization using Dabsyl chloride as reagent. It includes the tools and reagents for vapor-phase hydrolysis by hydrochloric acid for proteins or peptides, and by using such a DAB-Label kit, collagen can be hydrolyzed and the amino acids can be derivatized with Dabsyl chloride in order to analyze the constituent amino acids.
In this report, collagen was hydrolyzed with the DAB-Label kit and the derivatized amino acids were separated by Ultra High-performance Liquid Chromatography (UHPLC) using UV detection.

Experimental
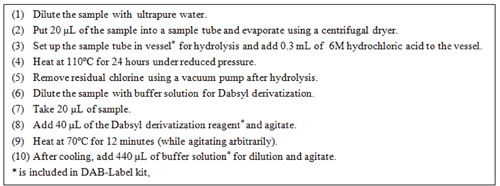
The procedure for hydrolysis and Dabsyl derivatization is shown in figure 1.
Keywords
430024XRE
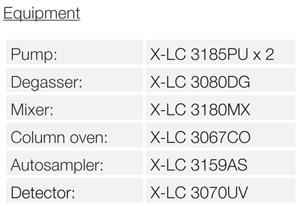
Results
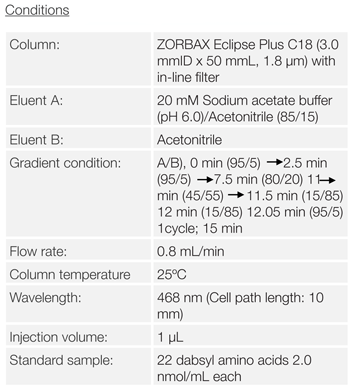
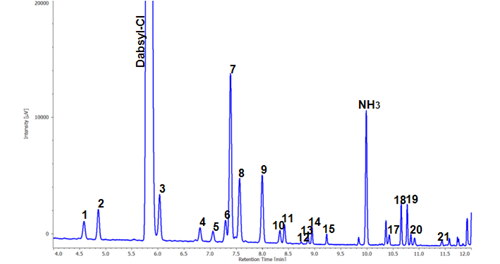
The chromatogram of 22 constituents of Dabsyl amino acids in a standard mixture is shown in figure 2. Twenty-two constituents of amino acids were clearly separated within 11.5 minutes with gradient elution for sufficient separation of hydroxyproline and hydroxylysine which are specific amino acids for collagen.
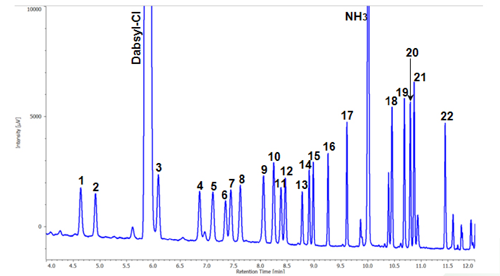
The chromatogram of the amino acids derivatized with Dabsyl after hydrolysis of a drink containing collagen is shown in figure 3. Amino acids including GABA and ornithine contained in the drink were successfully detected.
The peak numbers are the same as in figure 2.
Preparation: Hydrolysis and Dabsyl derivatization were implemented according to the procedure shown in figure 1.
Featured Products:

High Speed Analysis of Dabsyl Amino Acids in Collagen using UHPLC
Introduction
Collagen, one of the proteins that has been recently attracting attention from the viewpoint of health and beauty, is a main constituent of corium which gives skin vitality and keeps the skin soft without wrinkles. The amino acid structure of collagen is an arrangement of glycine sequencing at every 3rd residue and contains other amino acids such as proline, hydroxyproline and alanine. In addition hydroxyproline and hydroxylysine are present in collagen. In order to measure the constituent amino acids of proteins or peptides such as collagen, sample preparation such as hydrolysis is required. A DAB-Label kit is available from JASCO for amino acid derivatization using Dabsyl chloride as reagent. It includes the tools and reagents for vapor-phase hydrolysis by hydrochloric acid for proteins or peptides, and by using such a DAB-Label kit, collagen can be hydrolyzed and the amino acids can be derivatized with Dabsyl chloride in order to analyze the constituent amino acids.
In this report, collagen was hydrolyzed with the DAB-Label kit and the derivatized amino acids were separated by Ultra High-performance Liquid Chromatography (UHPLC) using UV detection.

Experimental


The procedure for hydrolysis and Dabsyl derivatization is shown in figure 1.

Results
The chromatogram of 22 constituents of Dabsyl amino acids in a standard mixture is shown in figure 2. Twenty-two constituents of amino acids were clearly separated within 11.5 minutes with gradient elution for sufficient separation of hydroxyproline and hydroxylysine which are specific amino acids for collagen.

The chromatogram of the amino acids derivatized with Dabsyl after hydrolysis of a drink containing collagen is shown in figure 3. Amino acids including GABA and ornithine contained in the drink were successfully detected.

The peak numbers are the same as in figure 2.
Preparation: Hydrolysis and Dabsyl derivatization were implemented according to the procedure shown in figure 1.
Keywords
430024XRE

 Download This Application
Download This Application

