High Speed Separation of Polycyclic Aromatic Hydrocarbons by X-LC
January 5, 2024
Introduction
Polycyclic aromatic hydrocarbons (PAHs), which consist of fused aromatic rings, are produced by incomplete combustion of carbon-containing fuels such as diesel and coal. Some of them are suspected to be carcinogens, requiring the analysis of environmental PAHs. In this paper, we examine the utility of the JASCO X-LC system for the separation and determination of PAHs.
Experimental

The UHPLC system utilized in this experiment was a JASCO X-LC system consisting of two PU-3185 pumps, a DG-3080 mobile phase degasser, a MX-3080 mixing unit, a CO-3067 column oven, an FP-3120 fluorescence detector, an AS-3059 autosampler, and the ChromNAV chromatography data system. The column used is a Zorbax Eclipse PAH (2.1 mm ID x 50 mmL, 1.8 µm). PAHs from the residue in a diesel engine was extracted for 8 hours using a soxhlet apparatus with dichloromethane as the extraction solvent.
Keywords
230005X
Results
Figure 1 is an X-LC chromatogram of a standard mixture of 15 components of PAHs (200 pg each). The chromatograms were obtained using gradient elution and a time program of excitation and emission wavelengths for the fluorescence detector. The X-LC system provides an analysis time 3X shorter than a conventional HPLC separation. Figure 2 outlines an X-LC chromatogram obtained from the diesel engine residue with eight components separated with satisfactory resolution.
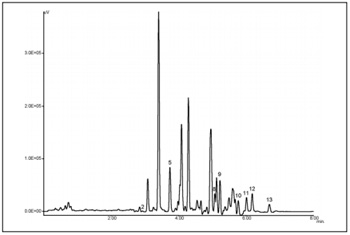
PAHs were extracted using a soxhlet apparatus as outlined within the text.
The chromatographic/detection conditions are the same as used in Figure 1.
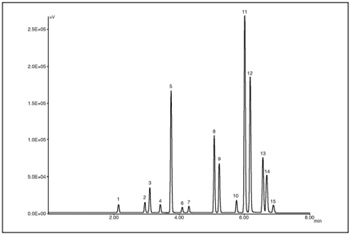
Peak: 1 = Naphthalene, 2 = Acenaphthene, 3 = Fluorene, 4 = Phenanthrene, 5 = Anthracene, 6 = Fluoranthene, 7 = Pyrene, 8 = Benzo(a)anthracene, 9 = Chrysene, 10 = Benzo(b)fluoranthene, 11 = Benzo(k)fluoranthene, 12 = Benzo(a)pyrene, 13 = Dibenzo(a,h)anthracene, 14 = Benzo(g,h,i)perylene, 15 = Indeno(1,2,3-c,d)pyrene (200 pg each). Chromatographic conditions: column = Zorbax Eclipse PAH(2.1 mmID. x 50 mmL., 1.8 µm); mobile phase = A(water), B(acetonitrile): A/B(60/40, 0 min); A/B(32/68, 3.5 min); A/B(0/100, 6 min); A/B(0/100, 8.2 min); A/B(60/40, 8.3 min); flow rate = 0.6 mL/min; column temperature = 30°C, wavelength = Ex/Em: (280 nm / 330 nm, 0 min); (260 nm / 340 nm, 2.65min); (250 nm / 420 nm, 3.55 min); (270 nm / 400 nm, 4.6 min); (295 nm / 410 nm, 5.9 min); (290 nm / 500 nm, 6.8 min); injection volume = 1 µL.
Featured Products:

High Speed Separation of Polycyclic Aromatic Hydrocarbons by X-LC
Introduction
Polycyclic aromatic hydrocarbons (PAHs), which consist of fused aromatic rings, are produced by incomplete combustion of carbon-containing fuels such as diesel and coal. Some of them are suspected to be carcinogens, requiring the analysis of environmental PAHs. In this paper, we examine the utility of the JASCO X-LC system for the separation and determination of PAHs.
Experimental

The UHPLC system utilized in this experiment was a JASCO X-LC system consisting of two PU-3185 pumps, a DG-3080 mobile phase degasser, a MX-3080 mixing unit, a CO-3067 column oven, an FP-3120 fluorescence detector, an AS-3059 autosampler, and the ChromNAV chromatography data system. The column used is a Zorbax Eclipse PAH (2.1 mm ID x 50 mmL, 1.8 µm). PAHs from the residue in a diesel engine was extracted for 8 hours using a soxhlet apparatus with dichloromethane as the extraction solvent.
Keywords
230005X
Results
Figure 1 is an X-LC chromatogram of a standard mixture of 15 components of PAHs (200 pg each). The chromatograms were obtained using gradient elution and a time program of excitation and emission wavelengths for the fluorescence detector. The X-LC system provides an analysis time 3X shorter than a conventional HPLC separation. Figure 2 outlines an X-LC chromatogram obtained from the diesel engine residue with eight components separated with satisfactory resolution.

PAHs were extracted using a soxhlet apparatus as outlined within the text.
The chromatographic/detection conditions are the same as used in Figure 1.

Peak: 1 = Naphthalene, 2 = Acenaphthene, 3 = Fluorene, 4 = Phenanthrene, 5 = Anthracene, 6 = Fluoranthene, 7 = Pyrene, 8 = Benzo(a)anthracene, 9 = Chrysene, 10 = Benzo(b)fluoranthene, 11 = Benzo(k)fluoranthene, 12 = Benzo(a)pyrene, 13 = Dibenzo(a,h)anthracene, 14 = Benzo(g,h,i)perylene, 15 = Indeno(1,2,3-c,d)pyrene (200 pg each). Chromatographic conditions: column = Zorbax Eclipse PAH(2.1 mmID. x 50 mmL., 1.8 µm); mobile phase = A(water), B(acetonitrile): A/B(60/40, 0 min); A/B(32/68, 3.5 min); A/B(0/100, 6 min); A/B(0/100, 8.2 min); A/B(60/40, 8.3 min); flow rate = 0.6 mL/min; column temperature = 30°C, wavelength = Ex/Em: (280 nm / 330 nm, 0 min); (260 nm / 340 nm, 2.65min); (250 nm / 420 nm, 3.55 min); (270 nm / 400 nm, 4.6 min); (295 nm / 410 nm, 5.9 min); (290 nm / 500 nm, 6.8 min); injection volume = 1 µL.

 Download This Application
Download This Application

