Semi-Preparative Separation of Glycyrrhizic Acid in Glycyrrhiza
August 19, 2022
Introduction
Glycyrrhiza is a known source of natural medicine belonging to the legume family (Fabaceae) and has excellent beneficial effects as an analgesic, anti-inflammatory, anti-gastralgia, antitussive/expectorant and detoxification and is also used as a cold medicine. Glycyrrhizic acid contained richly in Glycyrrhiza is several tens to hundred times sweeter than sucrose and is used as natural sweetener.
In this LC application data, after studying the separation of Glycyrrhizic acid from the extract of Glycyrrhiza powder using conventional HPLC system, the separation using scaled-up semi-preparative HPLC will be reported.
Experimental
Equipment
| Conventional HPLC | |
|---|---|
| Eluent Pump: | PU-2089 |
| Autosampler: | AS-2057 |
| Column oven: | CO-2060 |
| Detector: | MD-2018 |
| Semi-Preparative HPLC | |
|---|---|
| Eluent Pump: | PU-2086 |
| Autosampler: | AS-2058 |
| Column oven: | CO-2060 |
| Detector: | MD-2018 |
| (with semi-prep. cell) | |
| Chromatography data system: | ChromNAV |
| Fraction collector: | ADVANTEC SCF 122SC |
| Fraction collector controller: | FC-2088-30 |
Conditions
| Conventional HPLC | |
|---|---|
| Column: | YMC-PACK Pro C18 |
| (4.6 mm ID x 250 mmL, 5 µm) | |
| Eluent: | Acetonitrile / Water (35/65) |
| Eluent flow rate: | 1.0 mL/min |
| Column temp.: | 25°C |
| Wavelength: | 254 nm |
| Injection volume: | 200 µL |
| Standard sample: | Powdered Glycyrrhiza |
| (0.5 g/100 mL in 50% ethanol) |
| Semi-Preparative HPLC | |
|---|---|
| Column: | YMC-PACK Pro C18 |
| (20 mm ID x 250 mmL, 5 µm) | |
| Eluent: | Acetonitrile / Water (35/65) |
| Eluent flow rate: | 15 mL/min |
| Column temp.: | 25°C |
| Wavelength: | 254 nm |
| Injection volume: | 5 mL |
| Standard sample: | Powdered Glycyrrhiza |
| (0.5 g/100 mL in 50% ethanol) |
Preparation (extraction)
- Weigh 0.5 g of powdered glycyrrhiza and place in a centrifuge tube.
- Add 60 mL of diluted ethanol solution (ethanol/Water (50/50)) and mix for 15 minutes.
- Centrifuge (2000 rpm, 10 minutes) and decant the supernatant into a100 mL volumetric flask.
- Add 30 mL of diluted ethanol solution (ethanol/Water (50/50)) to the residue and repeat the procedure.
- Add diluted ethanol solution to the collected supernatant in the volumetric flask and make up to100 mL.
Keywords
Analytical HPLC, Semi-preparative HPLC, Glycyrrhiza, Glycyrrhizic acid, Neutraceutical, Natural medicine
Results
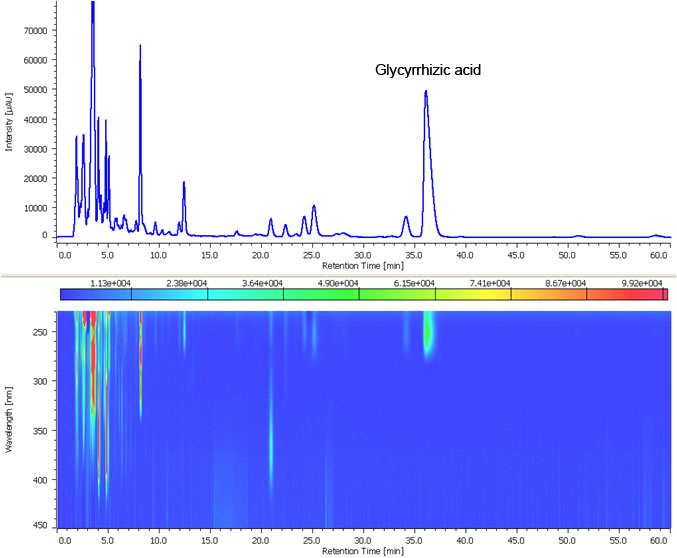
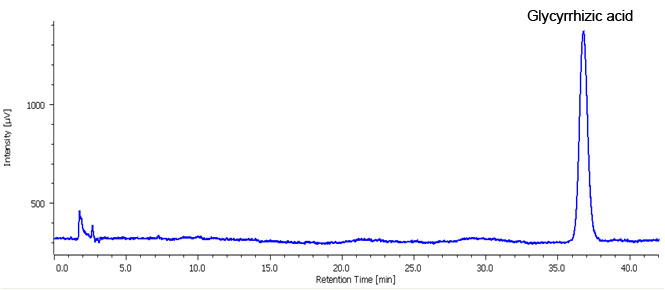
Fig. 2. shows chromatogram and contour plot of the extract from Glycyrrhiza powder acquired using analytical HPLC. Using the MD-2018 PDA detector and using spectral comparison the separation was optimized to resolve the target from the other components, optimal separation was obtained in 40 minutes.
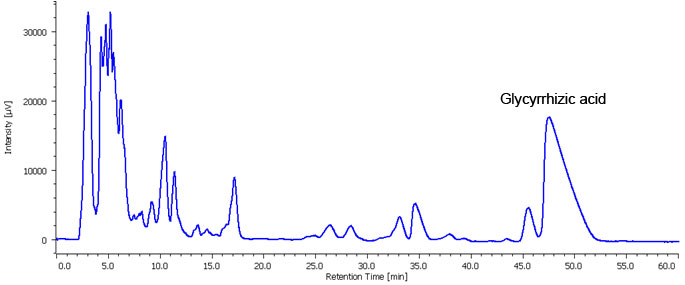
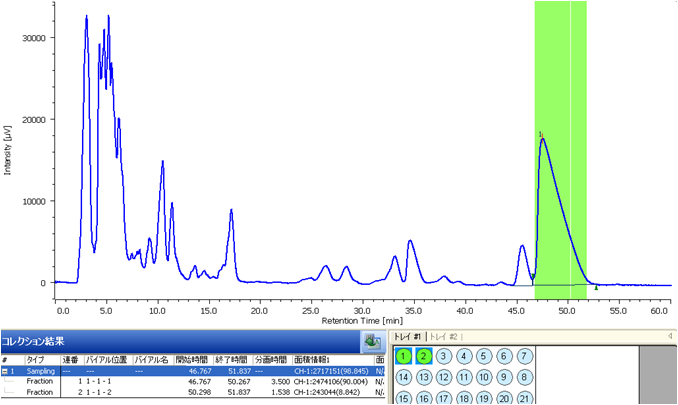
Fig. 3. shows the chromatogram using semi-preparative separation scaled-up from analytical. To maximize the recovery of Glycyrrhizic acid a 5 mL sample volume was injected. As shown in Figure 3, the column loading was increased but at the sacrifice of peak shape. Fig. 4. shows the fraction display in the ChromNAV Chromatography data system. The peaks fraction and sample rack positions for the target are highlighted in green. The sample peak was collected into two vials. Figure 5. shows a chromatogram of the purity check of this fraction using the same conditions as in Figure 2. It is confirmed that Glycyrrhizic acid was isolated as single component.
Featured Products:

Semi-Preparative Separation of Glycyrrhizic Acid in Glycyrrhiza
Introduction
Glycyrrhiza is a known source of natural medicine belonging to the legume family (Fabaceae) and has excellent beneficial effects as an analgesic, anti-inflammatory, anti-gastralgia, antitussive/expectorant and detoxification and is also used as a cold medicine. Glycyrrhizic acid contained richly in Glycyrrhiza is several tens to hundred times sweeter than sucrose and is used as natural sweetener.
In this LC application data, after studying the separation of Glycyrrhizic acid from the extract of Glycyrrhiza powder using conventional HPLC system, the separation using scaled-up semi-preparative HPLC will be reported.
Experimental
Equipment
| Conventional HPLC | |
|---|---|
| Eluent Pump: | PU-2089 |
| Autosampler: | AS-2057 |
| Column oven: | CO-2060 |
| Detector: | MD-2018 |
| Semi-Preparative HPLC | |
|---|---|
| Eluent Pump: | PU-2086 |
| Autosampler: | AS-2058 |
| Column oven: | CO-2060 |
| Detector: | MD-2018 |
| (with semi-prep. cell) | |
| Chromatography data system: | ChromNAV |
| Fraction collector: | ADVANTEC SCF 122SC |
| Fraction collector controller: | FC-2088-30 |
Conditions
| Conventional HPLC | |
|---|---|
| Column: | YMC-PACK Pro C18 |
| (4.6 mm ID x 250 mmL, 5 µm) | |
| Eluent: | Acetonitrile / Water (35/65) |
| Eluent flow rate: | 1.0 mL/min |
| Column temp.: | 25°C |
| Wavelength: | 254 nm |
| Injection volume: | 200 µL |
| Standard sample: | Powdered Glycyrrhiza |
| (0.5 g/100 mL in 50% ethanol) |
| Semi-Preparative HPLC | |
|---|---|
| Column: | YMC-PACK Pro C18 |
| (20 mm ID x 250 mmL, 5 µm) | |
| Eluent: | Acetonitrile / Water (35/65) |
| Eluent flow rate: | 15 mL/min |
| Column temp.: | 25°C |
| Wavelength: | 254 nm |
| Injection volume: | 5 mL |
| Standard sample: | Powdered Glycyrrhiza |
| (0.5 g/100 mL in 50% ethanol) |
Preparation (extraction)
- Weigh 0.5 g of powdered glycyrrhiza and place in a centrifuge tube.
- Add 60 mL of diluted ethanol solution (ethanol/Water (50/50)) and mix for 15 minutes.
- Centrifuge (2000 rpm, 10 minutes) and decant the supernatant into a100 mL volumetric flask.
- Add 30 mL of diluted ethanol solution (ethanol/Water (50/50)) to the residue and repeat the procedure.
- Add diluted ethanol solution to the collected supernatant in the volumetric flask and make up to100 mL.

Keywords
Analytical HPLC, Semi-preparative HPLC, Glycyrrhiza, Glycyrrhizic acid, Neutraceutical, Natural medicine
Results
Fig. 2. shows chromatogram and contour plot of the extract from Glycyrrhiza powder acquired using analytical HPLC. Using the MD-2018 PDA detector and using spectral comparison the separation was optimized to resolve the target from the other components, optimal separation was obtained in 40 minutes.
Fig. 3. shows the chromatogram using semi-preparative separation scaled-up from analytical. To maximize the recovery of Glycyrrhizic acid a 5 mL sample volume was injected. As shown in Figure 3, the column loading was increased but at the sacrifice of peak shape. Fig. 4. shows the fraction display in the ChromNAV Chromatography data system. The peaks fraction and sample rack positions for the target are highlighted in green. The sample peak was collected into two vials. Figure 5. shows a chromatogram of the purity check of this fraction using the same conditions as in Figure 2. It is confirmed that Glycyrrhizic acid was isolated as single component.





 Download This Application
Download This Application

