V-Series UV-visible/NIR spectrophotometer validation software
August 25, 2022
Introduction

When an analytical instrument is used in a regulated laboratory, a performance verification is often required to ensure that it is operating to the required specifications. The V-600 UV-Visible/NIR instruments are provided with a [Validation] application as part of Spectra Manager II Suite.
The V-600 instrument validation included with Spectra Manager II Suite provides a series of test protocols to verify that the instrument is performing to correct operating specifications. [Pass/Fail] criteria are established for each instrument based on individual specifications. The validation includes tests that use either intrinsic or external standard reference materials (with specifications to NIST or some other recognized authority).
Figure 1 outlines the various performance tests that can be selected within the software. The qualification tests that do not require external standards have been checked in the displayed software dialog. The unchecked qualification tests require external standards (optional, contact JASCO for more details). Specific tests complying with regulatory compliance include US Pharmacopeia (USP), European Pharmacopeia (EP) Japanese Pharmacopeia (JP) or the Japanese Industrial Standards (JIS). Custom tests can also be created to the user’s criteria by manually editing the SRM type and corresponding specification.
The tests can be easily performed and the only user input required are the Photometric Accuracy values (Figure 2) and Photometric Repeatability values for the external standards. All other performance tests have specified values applied according to the instrument type and the instrument parameters depend upon the individual test procedure (Figure 3).
When the validation procedure is started, the software performs each test sequentially, prompting the user when to insert the specific standards. When the testing is complete, a detailed report, including pass/fail results, data and instrument conditions are saved in an electronic format and printed (Figure 4).
The requirements for testing largely depend on the SOP established by the laboratory, however JASCO generally recommends performing these tests at least quarterly or semi-annually.
Conclusion
Spectra Manager II Suite includes an instrument Validation package that is used to evaluate the performance of a V-600 (or V-700) UV-Visible/NIR spectrophotometer. These performance tests can be selected and performed as required by the user to confirm optimum performance of the instrument.
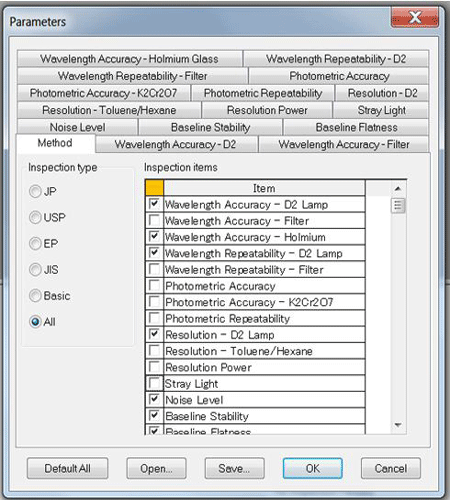
items require external standards (available as options).
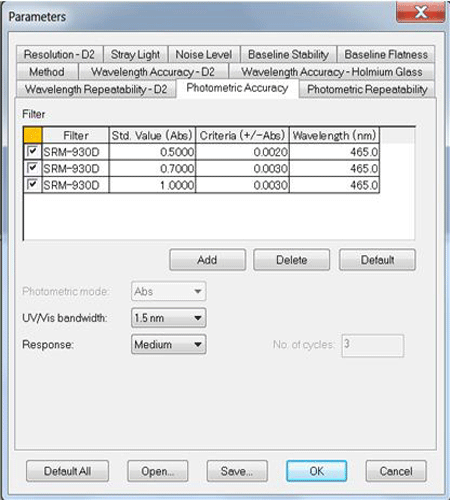
for each standard are input by the user based on the tested performance values for the standards.
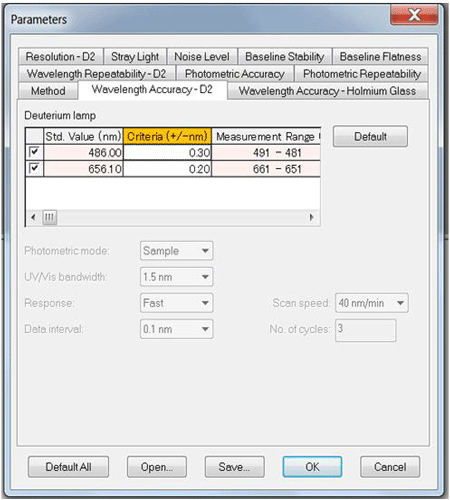
In this dialog, no inputs from the user are required.
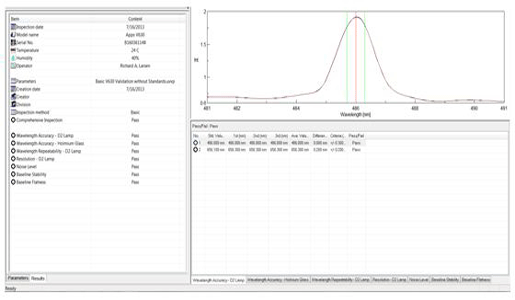
and the results can be saved electronically and/or printed.
Featured Products:
-
Film Holders for UV-Visible/NIR Spectrophotometers
-
MV-3000 Series Portable UV-Visible/NIR Spectrophotometer
-

Wide range UV-Visible/Near Infrared Spectrophotometer with PbS detector for wavelengths up to 3200nm
V-770 UV-Visible/NIR Spectrophotometer
-

A high sensitivity UV-Visible/NIR Spectrophotometer with InGaAs detector for wavelengths up to1600nm
V-780 UV-Visible/NIR Spectrophotometer

V-Series UV-visible/NIR spectrophotometer validation software
Introduction

When an analytical instrument is used in a regulated laboratory, a performance verification is often required to ensure that it is operating to the required specifications. The V-600 UV-Visible/NIR instruments are provided with a [Validation] application as part of Spectra Manager II Suite.
The V-600 instrument validation included with Spectra Manager II Suite provides a series of test protocols to verify that the instrument is performing to correct operating specifications. [Pass/Fail] criteria are established for each instrument based on individual specifications. The validation includes tests that use either intrinsic or external standard reference materials (with specifications to NIST or some other recognized authority).
Figure 1 outlines the various performance tests that can be selected within the software. The qualification tests that do not require external standards have been checked in the displayed software dialog. The unchecked qualification tests require external standards (optional, contact JASCO for more details). Specific tests complying with regulatory compliance include US Pharmacopeia (USP), European Pharmacopeia (EP) Japanese Pharmacopeia (JP) or the Japanese Industrial Standards (JIS). Custom tests can also be created to the user’s criteria by manually editing the SRM type and corresponding specification.
The tests can be easily performed and the only user input required are the Photometric Accuracy values (Figure 2) and Photometric Repeatability values for the external standards. All other performance tests have specified values applied according to the instrument type and the instrument parameters depend upon the individual test procedure (Figure 3).
When the validation procedure is started, the software performs each test sequentially, prompting the user when to insert the specific standards. When the testing is complete, a detailed report, including pass/fail results, data and instrument conditions are saved in an electronic format and printed (Figure 4).
The requirements for testing largely depend on the SOP established by the laboratory, however JASCO generally recommends performing these tests at least quarterly or semi-annually.
Conclusion
Spectra Manager II Suite includes an instrument Validation package that is used to evaluate the performance of a V-600 (or V-700) UV-Visible/NIR spectrophotometer. These performance tests can be selected and performed as required by the user to confirm optimum performance of the instrument.

items require external standards (available as options).

for each standard are input by the user based on the tested performance values for the standards.

In this dialog, no inputs from the user are required.

and the results can be saved electronically and/or printed.

 Download This Application
Download This Application
