Analysis of the effects of low temperature on the secondary structure of VHH antibodies
January 4, 2024
Introduction
VHH (variable domain heavy/heavy chain antibodies, lacking in light chains) single domain antibodies have high affinity, stability, productivity, and processability for antigens. These antibodies will highly likely offer a next-generation drug discovery modality. The storage environment may correspond to a loss of activity if the structure of a therapeutic antibody changes with temperature or freeze/thaw cycles. Therefore, it is essential to evaluate the structural stability in order to maintain quality control. Antibody based drugs are typically stored in a refrigerated or frozen state, but this may lead to denaturation from changes in temperature or repeated freezing and thawing.
FTIR spectroscopy has been widely used to evaluate the structure of proteins such as VHH antibodies. Secondary structure estimation (SSE) of α-Helices and β-Sheets can be performed quickly and easily. In this application note we discuss the results of FTIR measurements of secondary structure changes caused by the cooling process and repeated freezing and thawing of a VHH antibody that binds to the novel coronavirus (SARS-CoV-2).
Samples
Three Species of Anti SARS-CoV-2 VHH*1
No.1 (6.27 mg/mL)
No.2 (7.22 mg/mL)
No.3 (5.15 mg/mL)
Buffer: 20 mM PBS
Experimental
Structural change caused by the cooling process
An FT/IR-6600 spectrometer fitted with a Peltier controlled ATR PRO ONE (water-cooled) with a temperature range from room temperature to -10 ºC was used for the measurement (Figure 1). For sample No.1, IR spectra were measured at a range of temperatures from room temperature to just above freezing, and the secondary structure was estimated.

| Measurement Conditions | |||
| Main Unit | FT/IR-6600 | Accumulation | 1 minute |
| Attachment | ATR PRO ONE with cooling option | Filter | Band path filter (< 2000 cm-1) |
| Detector | Mid-band MCT | Purging Gas | N2 |
| Resolution | 4cm-1 | ||
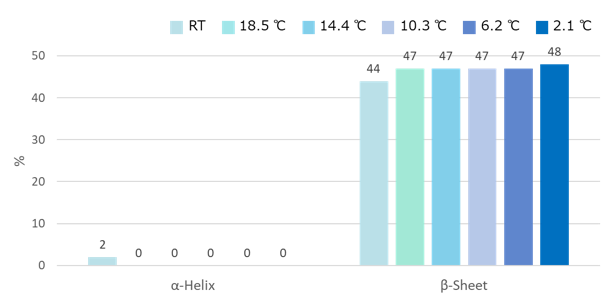
Figure 2 shows the percentage of α-Helix and β-Sheet structures at each temperature. The VHH antibody (sample No.1) is considered to have high stability during cooling, because the secondary structure does not change significantly during the cooling process.
Structural changes during repeated freezing and thawing
Samples Nos. 1, 2 and 3 were frozen to -25 ºC, thawed, and measured at room temperature. Freeze/thawing was repeated 3 times. The freezing time was set to 2 hours for the first cycle and 90 hours for the second and third cycles. In order to measure a small amount of sample with high sensitivity, a pentagonal prism multi-reflection ATR (ATR PRO PENTA)*2 was used (Figure 3.).

| Measurement Conditions | |||
| Spectrometer | FT/IR-6600 | Accumulation | 1 minute |
| Accessory | ATR PRO PENTA | Sample Volume | Several µL |
| Detector | MCT (PV type) | Purging Gas | Not used |
| Resolution | 4cm-1 | ||
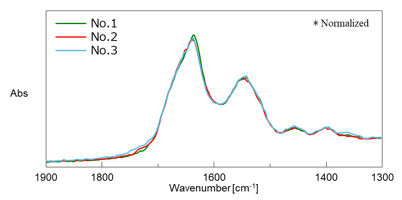
Figure 4 shows the difference spectrum between the sample and the buffer before freezing. The Amide I and Amide II bands were confirmed clearly even in a small amount (a few μL) of diluted aqueous protein solution.
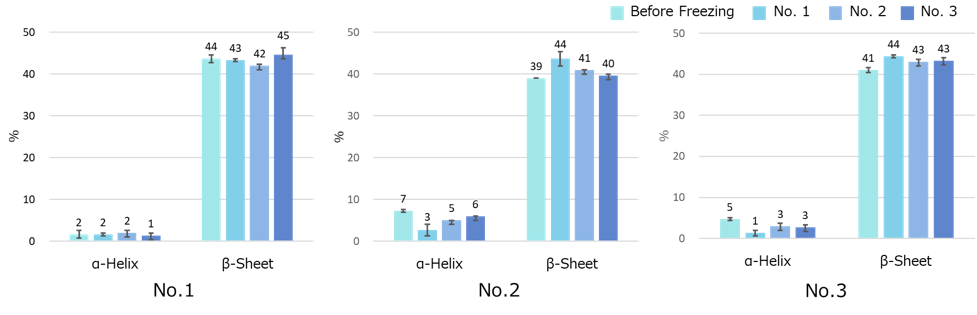
Figure 5 shows the SSE result using the ATR corrected difference spectrum. Numerical values in the figure are mean values for n = 3 and error bars are standard deviations. The secondary structures of the three VHH antibody samples used here were similar, and it was confirmed that the secondary structure of all three VHH antibodies did not change significantly after three freeze/thaw cycles.
Keywords
200-AT-0267, New corona virus, SARS-CoV-2, COVID-19, Biopharmaceuticals, Antibody drug, BioSimilar, VHH antibody, HOS, Secondary Structure, Tertiary Structure
Conclusion
It was found that the VHH antibody used in this experiment does not significantly change its secondary structure during the cooling process nor freezing and thawing, demonstrating high structural stability against cooling. We believe that similar systems can be used not only for stability of antibody drugs against environmental changes but also for other evaluations, such as reactivity with other components.
*1: VHH antibody was kindly provided by RePHAGEN https://rephagen.com/
*2: Application note 140-AT-0263 ‘Protein secondary structure analysis in low concentration aqueous solutions’
Required Products and Software
System Configuration
[Structural change during the cooling process]
| Model | Description | Part Number | |
| Main Unit | FT/IR-6600 | FT/IR Spectrometer | 7085-J002A*1) |
| Detector | MCT-6000M | Mid-band MCT detector with switching mechanism | 6888-J714A*2) |
| Attachment | ATR PRO ONE with cooling option | *3) | |
| Software | IR SSE-4000 | Secondary structure estimation program | 4880-7234A |
*1) The FT/IR-4600/4700 and The FT/IR-6700/6800 can also be used.
*2) Choose the MCT-4000M with the FT/IR-4600/4700.
*3) A custom software package.
[Structural change during repeated freezing and thawing]
| Model | Description | Part Number | |
| Main Unit | FT/IR-6600 | FT/IR Spectrometer | 7085-J002A*1) |
| Detector | MCT-6000PV | MCT(PV) detector | 6584-J345A*5) |
| Attachment | ATR PRO PENTA | Multi reflection ATR | 6584-J343A |
| Software | IR SSE-4000 | Secondary structure estimation program | 4880-7234A |
*4) The FT/IR-4600/4700 and The FT/IR-6700/6800 can also be used.
*5) Choose the MCT-4000M with the FT/IR-4600/4700.
Featured Products:

Analysis of the effects of low temperature on the secondary structure of VHH antibodies
Introduction
VHH (variable domain heavy/heavy chain antibodies, lacking in light chains) single domain antibodies have high affinity, stability, productivity, and processability for antigens. These antibodies will highly likely offer a next-generation drug discovery modality. The storage environment may correspond to a loss of activity if the structure of a therapeutic antibody changes with temperature or freeze/thaw cycles. Therefore, it is essential to evaluate the structural stability in order to maintain quality control. Antibody based drugs are typically stored in a refrigerated or frozen state, but this may lead to denaturation from changes in temperature or repeated freezing and thawing.
FTIR spectroscopy has been widely used to evaluate the structure of proteins such as VHH antibodies. Secondary structure estimation (SSE) of α-Helices and β-Sheets can be performed quickly and easily. In this application note we discuss the results of FTIR measurements of secondary structure changes caused by the cooling process and repeated freezing and thawing of a VHH antibody that binds to the novel coronavirus (SARS-CoV-2).
Samples
Three Species of Anti SARS-CoV-2 VHH*1
No.1 (6.27 mg/mL)
No.2 (7.22 mg/mL)
No.3 (5.15 mg/mL)
Buffer: 20 mM PBS
Experimental
Structural change caused by the cooling process
An FT/IR-6600 spectrometer fitted with a Peltier controlled ATR PRO ONE (water-cooled) with a temperature range from room temperature to -10 ºC was used for the measurement (Figure 1). For sample No.1, IR spectra were measured at a range of temperatures from room temperature to just above freezing, and the secondary structure was estimated.

| Measurement Conditions | |||
| Main Unit | FT/IR-6600 | Accumulation | 1 minute |
| Attachment | ATR PRO ONE with cooling option | Filter | Band path filter (< 2000 cm-1) |
| Detector | Mid-band MCT | Purging Gas | N2 |
| Resolution | 4cm-1 | ||

Figure 2 shows the percentage of α-Helix and β-Sheet structures at each temperature. The VHH antibody (sample No.1) is considered to have high stability during cooling, because the secondary structure does not change significantly during the cooling process.
Structural changes during repeated freezing and thawing
Samples Nos. 1, 2 and 3 were frozen to -25 ºC, thawed, and measured at room temperature. Freeze/thawing was repeated 3 times. The freezing time was set to 2 hours for the first cycle and 90 hours for the second and third cycles. In order to measure a small amount of sample with high sensitivity, a pentagonal prism multi-reflection ATR (ATR PRO PENTA)*2 was used (Figure 3.).

| Measurement Conditions | |||
| Spectrometer | FT/IR-6600 | Accumulation | 1 minute |
| Accessory | ATR PRO PENTA | Sample Volume | Several µL |
| Detector | MCT (PV type) | Purging Gas | Not used |
| Resolution | 4cm-1 | ||

Figure 4 shows the difference spectrum between the sample and the buffer before freezing. The Amide I and Amide II bands were confirmed clearly even in a small amount (a few μL) of diluted aqueous protein solution.

Figure 5 shows the SSE result using the ATR corrected difference spectrum. Numerical values in the figure are mean values for n = 3 and error bars are standard deviations. The secondary structures of the three VHH antibody samples used here were similar, and it was confirmed that the secondary structure of all three VHH antibodies did not change significantly after three freeze/thaw cycles.
Conclusion
It was found that the VHH antibody used in this experiment does not significantly change its secondary structure during the cooling process nor freezing and thawing, demonstrating high structural stability against cooling. We believe that similar systems can be used not only for stability of antibody drugs against environmental changes but also for other evaluations, such as reactivity with other components.
*1: VHH antibody was kindly provided by RePHAGEN https://rephagen.com/
*2: Application note 140-AT-0263 ‘Protein secondary structure analysis in low concentration aqueous solutions’
Keywords
200-AT-0267, New corona virus, SARS-CoV-2, COVID-19, Biopharmaceuticals, Antibody drug, BioSimilar, VHH antibody, HOS, Secondary Structure, Tertiary Structure
Required Products and Software
System Configuration
[Structural change during the cooling process]
| Model | Description | Part Number | |
| Main Unit | FT/IR-6600 | FT/IR Spectrometer | 7085-J002A*1) |
| Detector | MCT-6000M | Mid-band MCT detector with switching mechanism | 6888-J714A*2) |
| Attachment | ATR PRO ONE with cooling option | *3) | |
| Software | IR SSE-4000 | Secondary structure estimation program | 4880-7234A |
*1) The FT/IR-4600/4700 and The FT/IR-6700/6800 can also be used.
*2) Choose the MCT-4000M with the FT/IR-4600/4700.
*3) A custom software package.
[Structural change during repeated freezing and thawing]
| Model | Description | Part Number | |
| Main Unit | FT/IR-6600 | FT/IR Spectrometer | 7085-J002A*1) |
| Detector | MCT-6000PV | MCT(PV) detector | 6584-J345A*5) |
| Attachment | ATR PRO PENTA | Multi reflection ATR | 6584-J343A |
| Software | IR SSE-4000 | Secondary structure estimation program | 4880-7234A |
*4) The FT/IR-4600/4700 and The FT/IR-6700/6800 can also be used.
*5) Choose the MCT-4000M with the FT/IR-4600/4700.

 Download This Application
Download This Application
