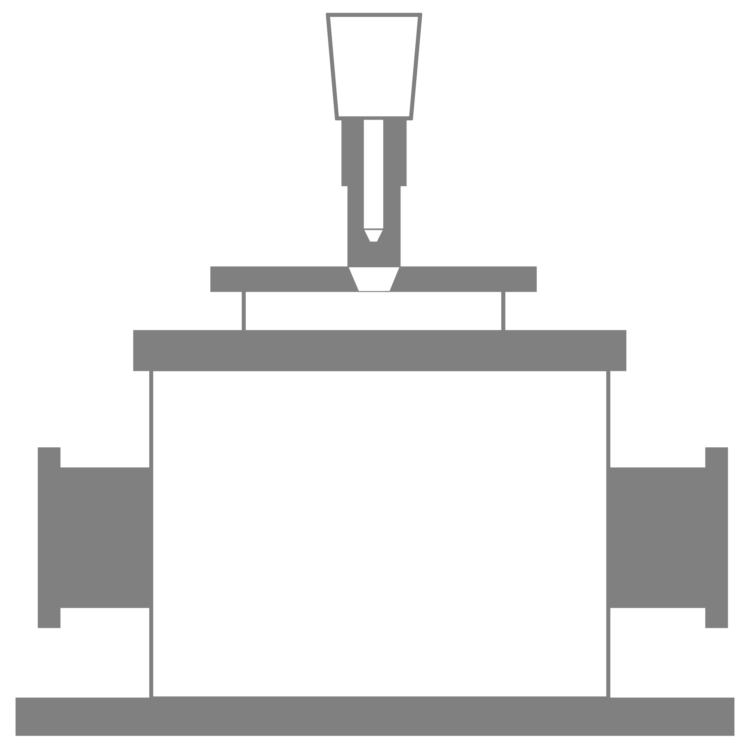
The ATR PRO PENTA X is a highly sensitive multi-bounce ATR accessory that can measure low-concentration aqueous solutions of 0.1% or less using only a small amount of sample (several µL) by adopting a uniquely designed 14-reflection Ge prism.
Features
-
Secondary Structure Estimation
Measure protein aqueous solutions to estimate the percentage of α-Helix, β-Sheet, β-Turn, and Other by deconvolving the amide I band.
-
Low Concentration Samples
In combination with a highly sensitive MCT detector, low concentration samples of 0.1% or less can be measured for quantitative analysis.
-
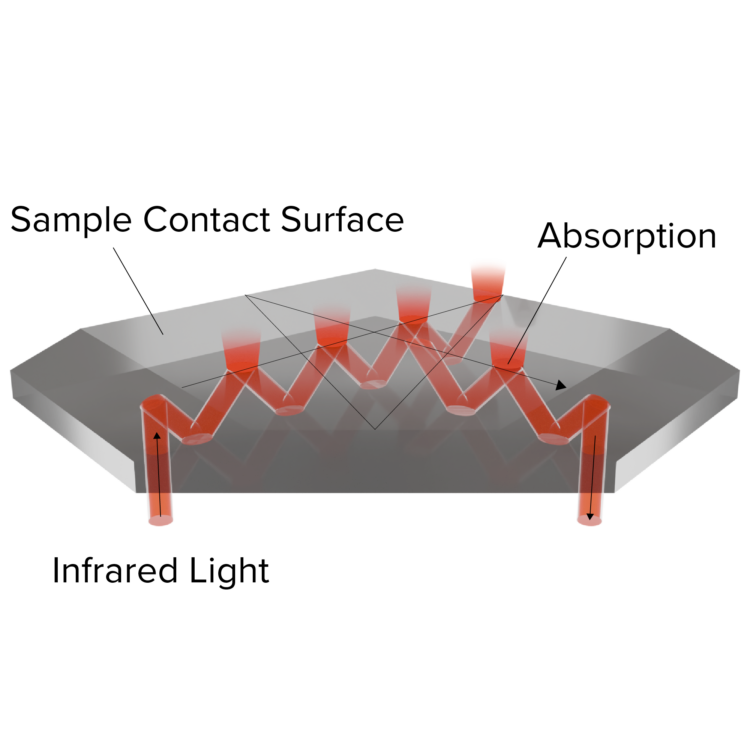
Unique Prism Shape
The unique pentagonal prism shape has an optical path with 14 bounces, increasing the effective penetration depth to significantly improve the signal-to-noise ratio.
-

Minimal Sample Volume
Liquid samples can be measured using only a small amount of sample (several µl).

Automatic Recognition on any X-Series FT/IR
Measurement Examples
Quantitation of low-concentration solutions
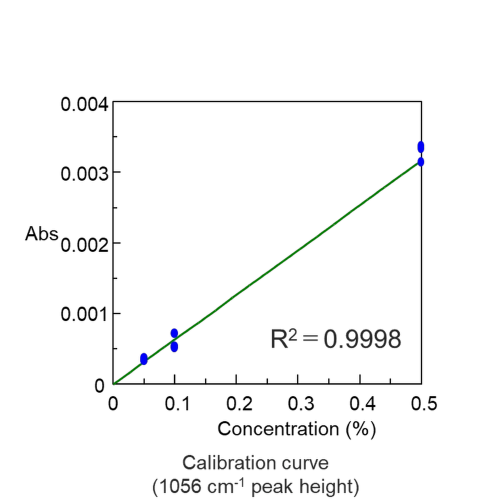
The spectra of 0.5%, 0.1%, and 0.05% sucrose aqueous solutions are shown in Figure 3. The spectra in Figure 3 are the resulting difference spectra from subtracting the spectrum of water from the spectrum of the sucrose aqueous solution. The calibration curve for the concentration of sucrose aqueous solutions shows a very high linearity (Figure 4), suggesting that the ATR PRO PENTA X is sufficient for the quantification of low-concentration solutions that were previously untestable.
*The quantification limit for the sucrose aqueous solution in this experiment was 0.042%, and the detection limit was 0.014%.
-
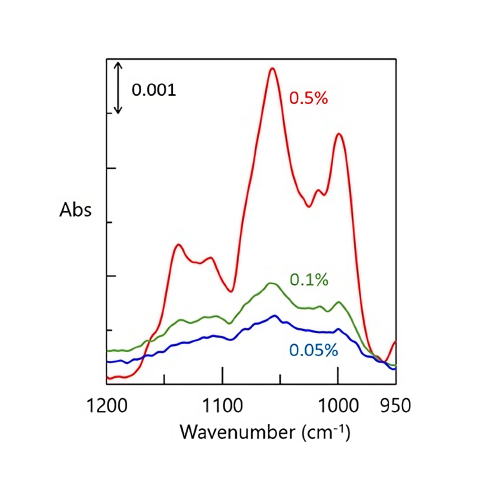
Difference spectrum of each sucrose aqueous solution.
-
Secondary structure analysis of proteins in low-concentration samples
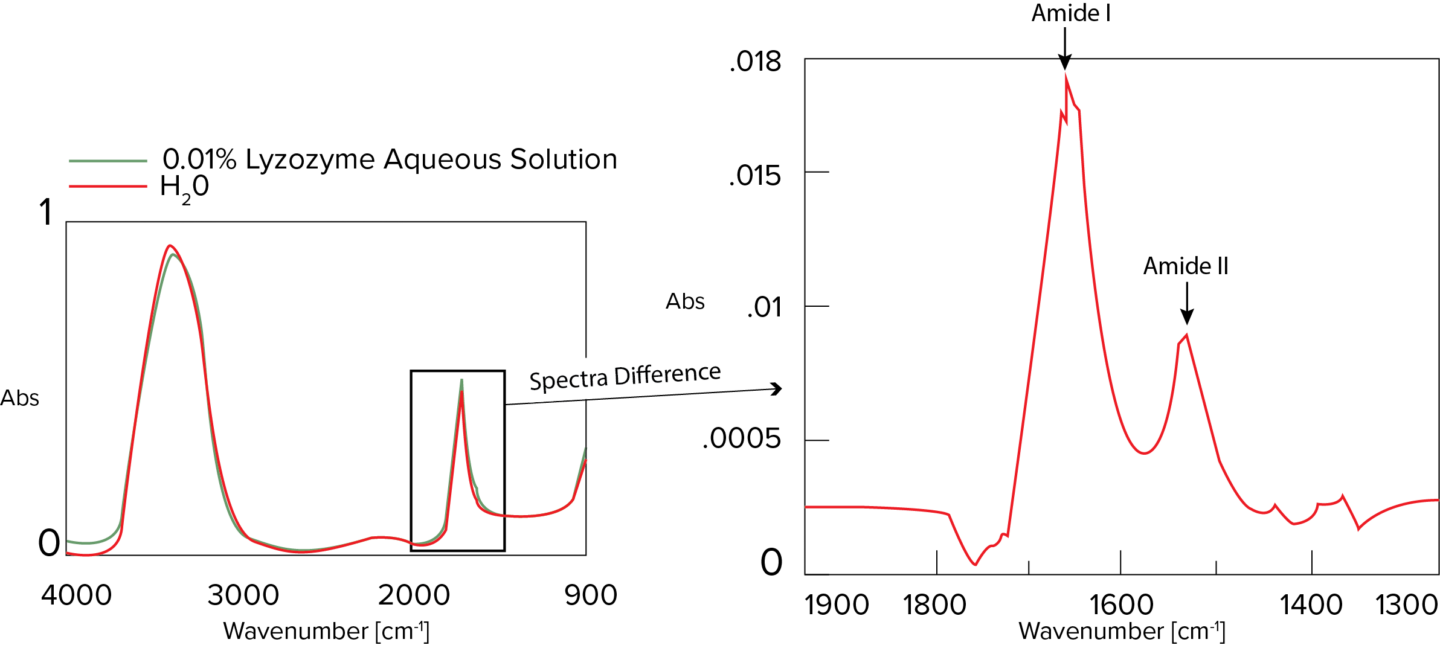
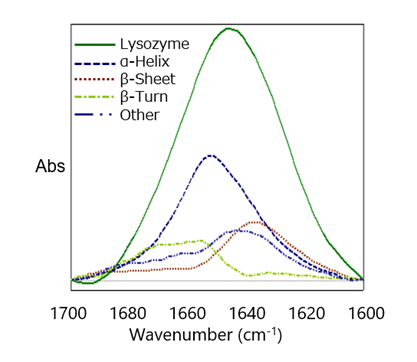
IR-SSE (IR protein secondary structure analysis) was performed using the difference spectrum of 0.01% lysozyme aqueous solution (Figure 5). Even at a low concentration of 0.01%, the peaks of amide I and amide II are clearly separated, and the peak shape is clear, so amide I information can be used for secondary structural analysis. Detection of lysozyme at a concentration of 0.01% was possible, and SSE was performed based on the Amide I band in the difference spectrum. Figure 6 shows the resulting concentration values. The results of the secondary structure analysis of the difference spectra measured using a heavy aqueous solution were reasonably consistent with those obtained by Sarver and his team [1].
[1] Sarver, R. W., Krueger, W.C., 1991. Anal. Biochem., 194, 89-100.
SSE Quantitation
| Structure | Percentage (%) |
|---|---|
| α-Helix | 40 |
| β-Sheet | 22 |
| β-Turn | 18 |
| Other | 20 |
Specifications
ATR Prism | Ge |
ATR/Sample Contact Area | - |
No. of Reflections | 14 |
Angle of Incidence | 45° |
Related Reading
-
Application
Protein secondary structure analysis in low concentration aqueous solutions
Infrared spectroscopy is an effective technique for component analysis and interaction analysis of biological samples such as proteins, sugars and lipids. FTIR spectroscopy can be used to measure solid and liquid samples when applied to secondary structure analysis of proteins. JASCO has previously reported the study of aqueous protein solutions*1....
-
Application
Analysis of the effects of low temperature on the secondary structure of VHH antibodies
VHH (variable domain heavy/heavy chain antibodies, lacking in light chains) single domain antibodies have high affinity, stability, productivity, and processability for antigens. These antibodies will highly likely offer a next-generation drug discovery modality. The storage environment may correspond to a loss of activity if the structure of a the...
Related Products
-
FT/IR-4X Spectrometer
The FT/IR-4X is a powerful Mid-IR FTIR spectrometer, with many features that you find in a research grade instrument, such as non-hygroscopic KRS-5 windows to prevent damage to the interferometer, a temperature controlled DLaTGS detector and a high output ceramic source for maximum sensitivity.
-
FT/IR-6X Spectrometer
The FT/lR-6X FTIR spectrometers with 28 degree Michelson interferometer and diode timing laser is an excellent choice for more demanding research applications, where better polarization performance or a more flexible approach to samples and measurement are required.
-
FT/IR-8X Spectrometer
The FT/IR-8X is a highly configurable optical system is applicable to virtually any FTIR application, from simple Mid IR measurement to much more complex analysis in the near and far IR.