Ion Chromatography of Chlorate Ions in Tap Water
August 11, 2022
Introduction
Chloric acid (Hydrogen chlorate), a strong oxidizing agent, occurs as a decomposition product derived from chlorine dioxide and sodium hypochlorite which are used as a disinfectant for water clarification.
We examined the usefulness of ion chromatography for the separation and determination of this compound.
Experimental

The system utilized in this experiment was a JASCO 2000 Plus system consisting of a PU-2080 pump, DG-2080-53 mobile phase degasser, CO-2060 column oven, AS-2057 autosampler, CD-5 conductivity detector(Shodex), an ion suppressor module, and ChromNAV chromatography data system.
The method was applied to the analysis of tap water. The water (1 L) was mixed with 0.5% ethylenediamine (1 mL), and then filtered with 0.2 µm membrane filter. An aliquot of 50 µL or 100 µL was injected.
Results
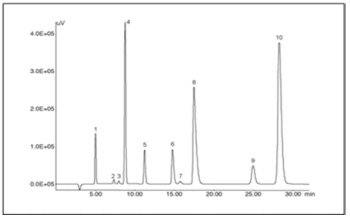
Figure 1 shows a chromatogram of a standard mixture of 10 anions. Resolution between Peak 7 (chlorate ion) and neighboring Peak 6 (bromide ion) exhibits greater than 2. There was no overlapping between these two peaks with any different concentrations, resulting in precise determination. Minimum detectable amounts proved 18.9 ppb (1.89 ng) at S/N=10, and 5.7 ppb (0.57 ng) at S/N=3. The calibration curve of chlorate ion indicated linearity between 5 ppb and 1 ppm with a coefficient of correlation of 1.000. Repetitive injection (n = 5) of 10 ppb solution of 100 µL provided relative standard deviation of 3.79%.
Peaks: 1 = fluoride (f, 2 ppm), 2 = chlorite (ClO2, 1 ppm), 3 = bromate (BrO3, 1 ppm), 4 = chloride (Cl, 10 ppm), 5 = nitrite (NO2, 5 ppm),
6 = bromide (Br, 10 ppm), 7 = chlorate (ClO3, 1 ppm), 8 = nitrate (NO3, 30 ppm), 9 = phosphate (PO43, 15 ppm),
10 = sulfate (SO42, 40 ppm) Chromatographic conditions: column = Shodex IC SI-52 4E (4.6 mm ID x 250 mm L);
column temperature = 45’C; mobile phase = 3.6 mM Na2CO3; flow rate=0.8 mL/min; injection volume=50 µL.
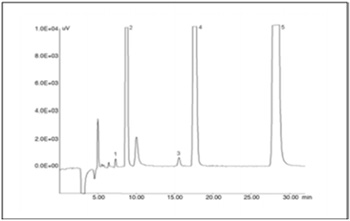
Figure 2 shows a chromatogram of the tap water mixed with chloric acid (60 ppb). Chlorate ion (Peak 3) is separated clearly from other peaks.
Peaks: 1 = chlorite (ClO2), 2 = chloride (Cl), 3 = chlorate (ClO3), 4 = nitrate (NO3), 5 = sulfate (SO42)
The other conditions are the same as in Figure 1 caption.
Featured Products:

Ion Chromatography of Chlorate Ions in Tap Water
Introduction
Chloric acid (Hydrogen chlorate), a strong oxidizing agent, occurs as a decomposition product derived from chlorine dioxide and sodium hypochlorite which are used as a disinfectant for water clarification.
We examined the usefulness of ion chromatography for the separation and determination of this compound.
Experimental

The system utilized in this experiment was a JASCO 2000 Plus system consisting of a PU-2080 pump, DG-2080-53 mobile phase degasser, CO-2060 column oven, AS-2057 autosampler, CD-5 conductivity detector(Shodex), an ion suppressor module, and ChromNAV chromatography data system.
The method was applied to the analysis of tap water. The water (1 L) was mixed with 0.5% ethylenediamine (1 mL), and then filtered with 0.2 µm membrane filter. An aliquot of 50 µL or 100 µL was injected.
Results
Figure 1 shows a chromatogram of a standard mixture of 10 anions. Resolution between Peak 7 (chlorate ion) and neighboring Peak 6 (bromide ion) exhibits greater than 2. There was no overlapping between these two peaks with any different concentrations, resulting in precise determination. Minimum detectable amounts proved 18.9 ppb (1.89 ng) at S/N=10, and 5.7 ppb (0.57 ng) at S/N=3. The calibration curve of chlorate ion indicated linearity between 5 ppb and 1 ppm with a coefficient of correlation of 1.000. Repetitive injection (n = 5) of 10 ppb solution of 100 µL provided relative standard deviation of 3.79%.

Peaks: 1 = fluoride (f, 2 ppm), 2 = chlorite (ClO2, 1 ppm), 3 = bromate (BrO3, 1 ppm), 4 = chloride (Cl, 10 ppm), 5 = nitrite (NO2, 5 ppm),
6 = bromide (Br, 10 ppm), 7 = chlorate (ClO3, 1 ppm), 8 = nitrate (NO3, 30 ppm), 9 = phosphate (PO43, 15 ppm),
10 = sulfate (SO42, 40 ppm) Chromatographic conditions: column = Shodex IC SI-52 4E (4.6 mm ID x 250 mm L);
column temperature = 45’C; mobile phase = 3.6 mM Na2CO3; flow rate=0.8 mL/min; injection volume=50 µL.
Figure 2 shows a chromatogram of the tap water mixed with chloric acid (60 ppb). Chlorate ion (Peak 3) is separated clearly from other peaks.

Peaks: 1 = chlorite (ClO2), 2 = chloride (Cl), 3 = chlorate (ClO3), 4 = nitrate (NO3), 5 = sulfate (SO42)
The other conditions are the same as in Figure 1 caption.

 Download This Application
Download This Application

