Comparison of Separation Behavior Among Silica-Based Columns in Supercritical Fluid Chromatography (SFC)
October 21, 2024
Introduction
Supercritical Fluid Chromatography (SFC) shares a similar separation mechanism with Normal Phase (NP) chromatography, a method used in High-Performance Liquid Chromatography (HPLC). In recent years, SFC has gained significant attention due to its ability to accelerate analyses while preserving high separation efficiency. This efficiency is largely attributed to the unique physical and chemical properties of supercritical CO2, which serves as the primary mobile phase solvent, offering both low viscosity and high diffusivity. However, no single column has emerged as the definitive first choice for separations in SFC, unlike the well-established and widely accepted C18 columns used in Reverse Phase (RP) chromatography. As a result, identifying a suitable column for separating specific target compounds in SFC often involves a method scouting process using multiple columns of different types.
In this application note, a method scouting experiment is presented for SFC using a single modifier solvent and six different types of columns. These columns, which have varying silica gel packing materials and modified functional groups, were tested for their ability to separate low-molecular-weight compounds with acidic, neutral, and basic properties. The separation behavior of these columns was assessed for five low-molecular-weight compounds and the key findings of the results are compared and discussed in this application note.*
*All data presented in this application note was obtained in collaboration with GL Sciences Inc.
Experimental
SFC System Configuration
| Liquid CO2 Pump | PU-4380 |
| Modifier Pump | PU-4180* |
| Autosampler | AS-4350 |
| Column Oven | CO-4065* |
| Photo Diode Array (PDA) Detector | MD-4010* |
| Back Pressure Regulator (BPR) | BP-4340 |
| *equipped with optional units | |
SFC Conditions
| Column | Silica-based columns (4.6 mm I.D. x 250 mm L, 5 µm, Details of the columns are described in Table 1.) |
| Mobile Phase A | Supercritical carbon dioxide (CO2) |
| Mobile Phase B | 0.1 % (w/v) ammonium acetate in methanol |
| Gradient | A/B = 95/5 (0.00 min) --> 60/40 (10.00 min) --> 95/5 (10.05 min), 1 cycle; 15 min |
| Flow Rate | 3.0 mL/min |
| Column Temperature | 40 ºC |
| Wavelength | 230 nm |
| Back Pressure | 10 MPa |
| Injection Volume | 5 µL |
| Sample Solution | Mixture of 5 low-molecular-weight compounds (200 µg/mL each in methanol) |
Structures of the Components in the Sample Solution
SFC Schematic Diagram
Keywords
Supercritical Fluid Chromatography, SFC, supercritical carbon dioxide, supercritical CO2, method scouting, separation behavior, Inertsil, InertSustain
Results
Using ChromNAV, a Chromatography Data System (CDS), comprehensive measurement conditions were generated for the combination of one modifier solvent and six different columns. The method scouting SFC system automatically switched between the six columns, enabling continuous measurements. The separation behavior of five low-molecular-weight compounds were assessed on each column.
Table 1 outlines the characteristics of the six columns used in this method scouting experiment.
Table 1. Types and Characteristics of Columns Used in this Application Note
| Column | Column Characteristics | End-Capping Treatment |
| Inertsil ODS-EP | An embedded column with octadecylsilane (ODS) groups chemically bonded to the silica gel of the stationary phase via a polar group. | without |
| Inertsil SIL-100A | A column with a silica gel stationary phase that has a pore size of 100 Å and extremely low metal content. | without |
| InertSustain NH2 | A column with amino-propyl groups chemically bonded to the silica gel of the stationary phase. | without |
| Inertsil ODS-HL | An ODS column with high planar recognition ability, with ODS groups bonded at high density to silica gel of the stationary phase. | with |
| Inertsil ODS-P | An ODS column with high planar recognition ability, which has polymerically bonded ODS groups to the silica gel of the stationary phase. It has a higher density of ODS groups bonded to the silica gel compared to the Inertsil ODS-HL column. | without |
| InertSustain AX-C18 | A mixed-mode column with reverse-phase and anion-exchange properties, which has ODS and alkylamine groups chemically bonded to the silica gel of the stationary phase. | with |
1: Caffeine, 2: Loxoprofen, 3: Amitriptyline, 4: Carbamazepine, 5: Salicylic acid
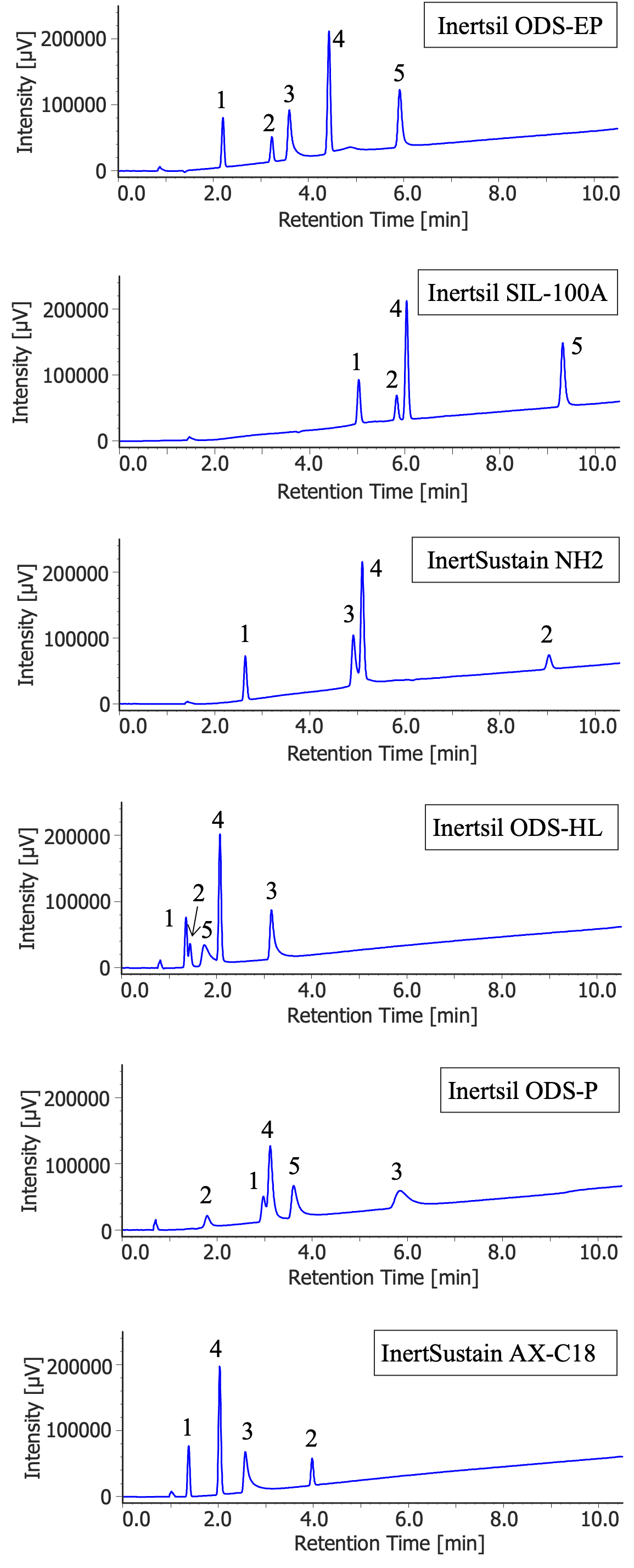
Figure 1 shows the chromatograms obtained from separating the five low-molecular-weight compounds on each column to compare the separation behavior using different silica columns.
The Inertsil ODS-EP column, with a polar group introduced at the base of the ODS group, highly retained polar acidic and/or basic compounds.
The Inertsil SIL-100A column has a similar separation behavior to the Inertsil ODS-EP column; however, overall retention was stronger with the Inertsil SIL-100A column. Amitriptyline did not elute under the conditions in this method because basic compounds are strongly adsorbed to the silanol group.
The InertSustain NH2 column is used as a NP column in HPLC, like the Inertsil SIL-100A column; however, due to being modified with basic functional groups, it tends to strongly retain acidic compounds. It was observed that the elution of the acidic compound, Loxoprofen, was significantly delayed compared to the Inertsil SIL-100A column, and salicylic acid did not elute from the column at all. On the other hand, the basic compound, amitriptyline, did elute from the column.
The Inertsil ODS-HL column has a high carbon content due to the densely bonded ODS groups and end-capping, leaving almost no residual silanol groups. Due to the absence of polar groups on the packing material surface, low-molecular-weight compounds, including the acidic and basic compounds measured in this application note, showed overall weak retention and eluted rapidly from the column.
The Inertsil ODS-P column has ODS groups bonded more densely than the Inertsil ODS-HL column and is not treated with end-capping like the Inertsil ODS-HL column. The presence of residual silanol groups on the packing material surface gives it similar retention strength to the Inertsil ODS-EP column; however, the overall peak shapes tend to be broader. The potential cause for the deterioration in peak shapes could be attributed to the extremely densely bonded ODS groups on the packing material of this column.
The InertSustain AX-C18 column is modified with two functional groups, ODS and tertiary amine. It shows a similar separation behavior to the InertSustain NH2 column, which is also modified with basic functional groups; however, there is a reversal in the elution order of carbamazepine and amitriptyline, and the overall retention strength is significantly different.
Conclusion
By conducting a method scouting experiment using one modifier solvent and six different columns, featuring unique silica gel packing materials and functional group modifications, distinct retention and separation behaviors for acidic, neutral, and basic low-molecular-weight compounds in SFC could be identified. This approach demonstrates that using a method scouting technique with multiple columns of different types in SFC is an effective and efficient way to quickly determine optimal analysis conditions for separating specific target compounds.
References
Author: LC Technical Solution Group
S. Iijima
Related Products

Comparison of Separation Behavior Among Silica-Based Columns in Supercritical Fluid Chromatography (SFC)
Introduction
Supercritical Fluid Chromatography (SFC) shares a similar separation mechanism with Normal Phase (NP) chromatography, a method used in High-Performance Liquid Chromatography (HPLC). In recent years, SFC has gained significant attention due to its ability to accelerate analyses while preserving high separation efficiency. This efficiency is largely attributed to the unique physical and chemical properties of supercritical CO2, which serves as the primary mobile phase solvent, offering both low viscosity and high diffusivity. However, no single column has emerged as the definitive first choice for separations in SFC, unlike the well-established and widely accepted C18 columns used in Reverse Phase (RP) chromatography. As a result, identifying a suitable column for separating specific target compounds in SFC often involves a method scouting process using multiple columns of different types.
In this application note, a method scouting experiment is presented for SFC using a single modifier solvent and six different types of columns. These columns, which have varying silica gel packing materials and modified functional groups, were tested for their ability to separate low-molecular-weight compounds with acidic, neutral, and basic properties. The separation behavior of these columns was assessed for five low-molecular-weight compounds and the key findings of the results are compared and discussed in this application note.*
*All data presented in this application note was obtained in collaboration with GL Sciences Inc.
Experimental
SFC System Configuration
| Liquid CO2 Pump | PU-4380 |
| Modifier Pump | PU-4180* |
| Autosampler | AS-4350 |
| Column Oven | CO-4065* |
| Photo Diode Array (PDA) Detector | MD-4010* |
| Back Pressure Regulator (BPR) | BP-4340 |
| *equipped with optional units | |
SFC Conditions
| Column | Silica-based columns (4.6 mm I.D. x 250 mm L, 5 µm, Details of the columns are described in Table 1.) |
| Mobile Phase A | Supercritical carbon dioxide (CO2) |
| Mobile Phase B | 0.1 % (w/v) ammonium acetate in methanol |
| Gradient | A/B = 95/5 (0.00 min) --> 60/40 (10.00 min) --> 95/5 (10.05 min), 1 cycle; 15 min |
| Flow Rate | 3.0 mL/min |
| Column Temperature | 40 ºC |
| Wavelength | 230 nm |
| Back Pressure | 10 MPa |
| Injection Volume | 5 µL |
| Sample Solution | Mixture of 5 low-molecular-weight compounds (200 µg/mL each in methanol) |
Structures of the Components in the Sample Solution
SFC Schematic Diagram
Results
Using ChromNAV, a Chromatography Data System (CDS), comprehensive measurement conditions were generated for the combination of one modifier solvent and six different columns. The method scouting SFC system automatically switched between the six columns, enabling continuous measurements. The separation behavior of five low-molecular-weight compounds were assessed on each column.
Table 1 outlines the characteristics of the six columns used in this method scouting experiment.
Table 1. Types and Characteristics of Columns Used in this Application Note
| Column | Column Characteristics | End-Capping Treatment |
| Inertsil ODS-EP | An embedded column with octadecylsilane (ODS) groups chemically bonded to the silica gel of the stationary phase via a polar group. | without |
| Inertsil SIL-100A | A column with a silica gel stationary phase that has a pore size of 100 Å and extremely low metal content. | without |
| InertSustain NH2 | A column with amino-propyl groups chemically bonded to the silica gel of the stationary phase. | without |
| Inertsil ODS-HL | An ODS column with high planar recognition ability, with ODS groups bonded at high density to silica gel of the stationary phase. | with |
| Inertsil ODS-P | An ODS column with high planar recognition ability, which has polymerically bonded ODS groups to the silica gel of the stationary phase. It has a higher density of ODS groups bonded to the silica gel compared to the Inertsil ODS-HL column. | without |
| InertSustain AX-C18 | A mixed-mode column with reverse-phase and anion-exchange properties, which has ODS and alkylamine groups chemically bonded to the silica gel of the stationary phase. | with |

1: Caffeine, 2: Loxoprofen, 3: Amitriptyline, 4: Carbamazepine, 5: Salicylic acid
Figure 1 shows the chromatograms obtained from separating the five low-molecular-weight compounds on each column to compare the separation behavior using different silica columns.
The Inertsil ODS-EP column, with a polar group introduced at the base of the ODS group, highly retained polar acidic and/or basic compounds.
The Inertsil SIL-100A column has a similar separation behavior to the Inertsil ODS-EP column; however, overall retention was stronger with the Inertsil SIL-100A column. Amitriptyline did not elute under the conditions in this method because basic compounds are strongly adsorbed to the silanol group.
The InertSustain NH2 column is used as a NP column in HPLC, like the Inertsil SIL-100A column; however, due to being modified with basic functional groups, it tends to strongly retain acidic compounds. It was observed that the elution of the acidic compound, Loxoprofen, was significantly delayed compared to the Inertsil SIL-100A column, and salicylic acid did not elute from the column at all. On the other hand, the basic compound, amitriptyline, did elute from the column.
The Inertsil ODS-HL column has a high carbon content due to the densely bonded ODS groups and end-capping, leaving almost no residual silanol groups. Due to the absence of polar groups on the packing material surface, low-molecular-weight compounds, including the acidic and basic compounds measured in this application note, showed overall weak retention and eluted rapidly from the column.
The Inertsil ODS-P column has ODS groups bonded more densely than the Inertsil ODS-HL column and is not treated with end-capping like the Inertsil ODS-HL column. The presence of residual silanol groups on the packing material surface gives it similar retention strength to the Inertsil ODS-EP column; however, the overall peak shapes tend to be broader. The potential cause for the deterioration in peak shapes could be attributed to the extremely densely bonded ODS groups on the packing material of this column.
The InertSustain AX-C18 column is modified with two functional groups, ODS and tertiary amine. It shows a similar separation behavior to the InertSustain NH2 column, which is also modified with basic functional groups; however, there is a reversal in the elution order of carbamazepine and amitriptyline, and the overall retention strength is significantly different.
Conclusion
By conducting a method scouting experiment using one modifier solvent and six different columns, featuring unique silica gel packing materials and functional group modifications, distinct retention and separation behaviors for acidic, neutral, and basic low-molecular-weight compounds in SFC could be identified. This approach demonstrates that using a method scouting technique with multiple columns of different types in SFC is an effective and efficient way to quickly determine optimal analysis conditions for separating specific target compounds.
Keywords
Supercritical Fluid Chromatography, SFC, supercritical carbon dioxide, supercritical CO2, method scouting, separation behavior, Inertsil, InertSustain
References
Author: LC Technical Solution Group
S. Iijima

 Download This Application
Download This Application