Fundamentals of Electronic Spectroscopy
Fundamentally electronic spectroscopy relies on the interaction of electromagnetic radiation, or light, with electrons. Typically, materials of interest have electrons in molecular orbitals which can be excited with UV or visible light (150 to 750 nm). For this reason, spectroscopy focused on light absorbed by electrons in a material is often denoted as “UV-Visible” spectroscopy. Likewise, the instruments focused on studying these phenomena are often referred to in shorthand as “UV-Vis” spectrometers. Most routinely UV-Vis spectroscopy is used for quantification, but critical information such as DNA melting temperatures or characterization of color and reflective properties can also be determined.
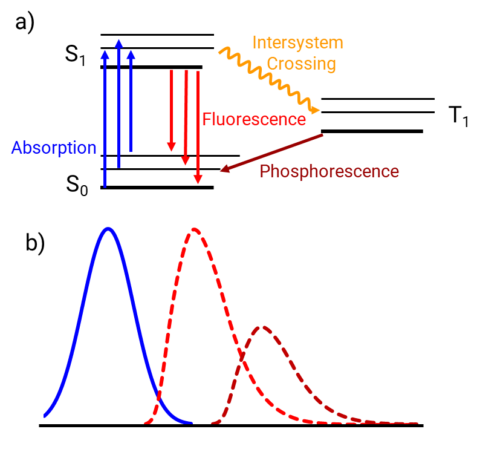
In addition to absorption by electrons, electronic spectroscopy focuses on the dissipation of that energy in the form of fluorescence or phosphorescence. These phenomena occur in a similar regime to absorption ranging form the UV to the visible. Unlike UV-Vis absorption, which requires a ratio of light intensity before and after the sample, fluorescence experiments use absolute measurement of the emitted signal leading to a higher degree of sensitivity. Furthermore, emission is more highly selective that absorption as not all materials are fluorescent or phosphorescent. Emission spectrometers can also be used to monitor phosphorescence, emission from the triplet state (T1).
For both absorption and fluorescence, the gap between ground and excited state electronic energy levels can sometimes be low enough to be detected in the near infrared region. Most often this is seen for metal bound materials, superstructures or materials that have charge-transfer character.
In the JASCO learning center we provide information on the theoretical methods for both fluorescence and absorption measurements. Additionally, we outline the general construction of the instruments that measure these phenomena and how to best use them through changing resolution, choosing cuvettes and solvent, and other experimental parameters.
