Acid Unfolding of Cytochrome C Measured with a Fluorescence Stopped-Flow Rapid-Kinetics System
January 5, 2024
Introduction
The fluorescence characteristics of tryptophan in proteins will vary depending on the structures surrounding the amino acid. The fluorescence of Cytochrome C is derived from the tryptophan in residue position 59. The natural state of this tryptophan is so close to the heme iron residue that the fluorescence is quenched by non-radiative energy transfer to the heme iron. When Cytochrome C is denatured by an acid, the distance between the tryptophan and heme iron changes and the fluorescence intensity increases. This application note demonstrates the measurement of the change in fluorescence intensity by acid denaturation of cytochrome C using a JASCO stopped-flow system.
Experimental

Emission (EM) spectra of lysozyme using an excitation (EX) of 280 nm are measured while controlling the temperature, to examine the effects of temperature change on the fluorescence. The model FP-6500 spectrofluorometer and an ETC-272 Peltier thermostatted cell holder are used for the measurements in this experiment, using the instrument parameters outlined below.
Measurement/Analysis Systems
- FP-6500 Spectrofluorometer
- SFS-482 Stopped-Flow system (Cell length: 10 mm)
- [Stopped-Flow Measurement] program
- [Reaction Rate Calculation] program
Syringe configuration
- S1: 10 mL, 0.5mg/mL cytochrome C
- S2: 10 mL, 0.1N sulfuric acid
Parameters
| Excitation Bandwidth: | 5 nm |
| Emission Bandwidth: | 5 nm |
| Response: | 2 seconds |
| Sensitivity: | Manual |
| Excitation Wavelength: | 280 nm |
| Emission Wavelength: | 340 nm |
| Measurement Range: | 0-5000 milliseconds |
| Measurement Interval: | 5 milliseconds |
| No. of Accumulations: | 4 |
| Flow Time: | 35 milliseconds |
| Flow Volume: | S1 = 100 µl; S2 = 100 µl |
| Mixing Ratio: | S1:S2 = 1:1 |
Figure 1 illustrates the measured and calculated results of the Cytochrome C emission during the stopped-flow experiment.
The measured data shows an large change in the fluorescence intensity corresponding to the acid denaturation of Cytochrome C. The stopped-flow system starts data acquisition before the syringe movement is completed to ensure that data can be monitored during the early stage of the reaction just before and after the sample and reactant have been combined.
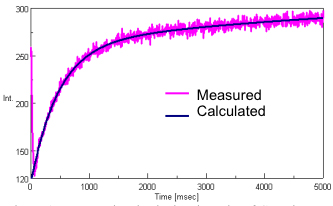
The reaction rate was calculated using the [Reaction Rate Calculation] program. The calculated range was 35 to 5000 msec and a 2-step reaction mechanism was applied to the calculation. The calculated results show an excellent fit to the experimental data.
| Calculation Range: | 35 to 5000 msec |
| Reaction Rate Formula: | Y(t) = -142.667 * exp(-t / 432.854) + -47.7112 * exp(-t / 3611.11) |
| Step 1 Time Constant: | 432.854 msec |
| Step 1 Rate Constant: | 0.00231025 msec-1 |
| Step 2 Time Constant: | 3611.11 msec |
| Step 2 Rate Constant: | 0.000276923 msec-1 |
Keywords
210-FP-0007
Required Products and Software
- FP-8300/8500/8600/8700 Spectrofluorometer
- SFS-852 Stopped-Flow Accessory
Featured Products:
-

Simple and sensitive system which readily accommodates routine measurements and accessories, such as spectral scanning, quantitation, and temperature control.
FP-8250 Spectrofluorometer
-

A powerful combination of performance, sensitivity and flexibility for biological, environmental and materials analysis.
FP-8350 Spectrofluorometer
-
FP-8650 NIR Spectrofluorometer

Acid Unfolding of Cytochrome C Measured with a Fluorescence Stopped-Flow Rapid-Kinetics System
Introduction
The fluorescence characteristics of tryptophan in proteins will vary depending on the structures surrounding the amino acid. The fluorescence of Cytochrome C is derived from the tryptophan in residue position 59. The natural state of this tryptophan is so close to the heme iron residue that the fluorescence is quenched by non-radiative energy transfer to the heme iron. When Cytochrome C is denatured by an acid, the distance between the tryptophan and heme iron changes and the fluorescence intensity increases. This application note demonstrates the measurement of the change in fluorescence intensity by acid denaturation of cytochrome C using a JASCO stopped-flow system.
Experimental

Emission (EM) spectra of lysozyme using an excitation (EX) of 280 nm are measured while controlling the temperature, to examine the effects of temperature change on the fluorescence. The model FP-6500 spectrofluorometer and an ETC-272 Peltier thermostatted cell holder are used for the measurements in this experiment, using the instrument parameters outlined below.
Measurement/Analysis Systems
- FP-6500 Spectrofluorometer
- SFS-482 Stopped-Flow system (Cell length: 10 mm)
- [Stopped-Flow Measurement] program
- [Reaction Rate Calculation] program
Syringe configuration
- S1: 10 mL, 0.5mg/mL cytochrome C
- S2: 10 mL, 0.1N sulfuric acid
Parameters
| Excitation Bandwidth: | 5 nm |
| Emission Bandwidth: | 5 nm |
| Response: | 2 seconds |
| Sensitivity: | Manual |
| Excitation Wavelength: | 280 nm |
| Emission Wavelength: | 340 nm |
| Measurement Range: | 0-5000 milliseconds |
| Measurement Interval: | 5 milliseconds |
| No. of Accumulations: | 4 |
| Flow Time: | 35 milliseconds |
| Flow Volume: | S1 = 100 µl; S2 = 100 µl |
| Mixing Ratio: | S1:S2 = 1:1 |
Figure 1 illustrates the measured and calculated results of the Cytochrome C emission during the stopped-flow experiment.
The measured data shows an large change in the fluorescence intensity corresponding to the acid denaturation of Cytochrome C. The stopped-flow system starts data acquisition before the syringe movement is completed to ensure that data can be monitored during the early stage of the reaction just before and after the sample and reactant have been combined.

The reaction rate was calculated using the [Reaction Rate Calculation] program. The calculated range was 35 to 5000 msec and a 2-step reaction mechanism was applied to the calculation. The calculated results show an excellent fit to the experimental data.
| Calculation Range: | 35 to 5000 msec |
| Reaction Rate Formula: | Y(t) = -142.667 * exp(-t / 432.854) + -47.7112 * exp(-t / 3611.11) |
| Step 1 Time Constant: | 432.854 msec |
| Step 1 Rate Constant: | 0.00231025 msec-1 |
| Step 2 Time Constant: | 3611.11 msec |
| Step 2 Rate Constant: | 0.000276923 msec-1 |
Keywords
210-FP-0007
Required Products and Software
- FP-8300/8500/8600/8700 Spectrofluorometer
- SFS-852 Stopped-Flow Accessory

 Download This Application
Download This Application