Fluorescence Depolarization Measurement of Liposome
May 1, 2024
Introduction

Fluorescence depolarization or fluorescence anisotropy can be observed when a fluorophore emits light of different intensities depending on the axes of polarization. When a molecule is excited by the absorption of light from its ground state to a transition state and relaxes to a stable vibrational state, it will then return back to its ground state by radiating fluorescence light. In this process there is a directionality, described by a transition dipole moment and determined by molecular structure, which is closely related to the direction of polarization of the excitation and fluorescence light.
For example, when fluorescence is emitted before a molecule rotates, the fluorescence light will be strongly polarized towards the direction of the excitation light’s polarization. If the light is emitted after the rotation of the molecule in a completely random direction, the fluorescence will be no longer polarized.
When measuring fluorescence polarization, the following factors will affect the molecular movement: (1) molecular size, (2) viscosity of the molecule’s environment, and (3) strength and degrees of freedom of a bound molecule. Larger molecules and more viscous or populated conditions require more energy in order for a molecule to move.
This application note will demonstrate the use of the FP-6500 spectrofluorometer and its automatic polarizers to monitor the temperature-dependent polarization of diphenyl hexatriene (DPH) upon addition to a lipid bilayer.
Measurement Conditions
| Excitation Wavelength | 357 nm | Emission Wavelength | 430 nm |
| Excitation Bandwidth | 3 nm | Emission Bandwidth | 3 nm |
| Response Time | 2 sec | Data Interval | 0.1 °C |
| Temperature Gradient | 20°C/hr | Measurement Mode | Fluorescence |
Experimental
The fluorescence polarization spectrum can be measured using the automatic polarizers. By placing the excitation polarizer in the vertical position and the emission polarizer in the horizontal and vertical positions, both fluorescence intensities, IVH and IVV, are measured, respectively. In order to correct for the sensitivity difference of the emission detector, the spectrum of rhodamine B is measured by setting the excitation polarizer in the horizontal position and setting the emission polarizer in the vertical and horizontal positions. The instrument grating factor is then defined as the following:
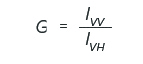
and multiplied by IVH to obtain an instrumental correction factor for the emission optics. The degree of polarization (P) is given by the following equation:
![]()
Keywords
210-FP-0016, FP-6500, Fluorescence, Polarization, Biochemistry
Results
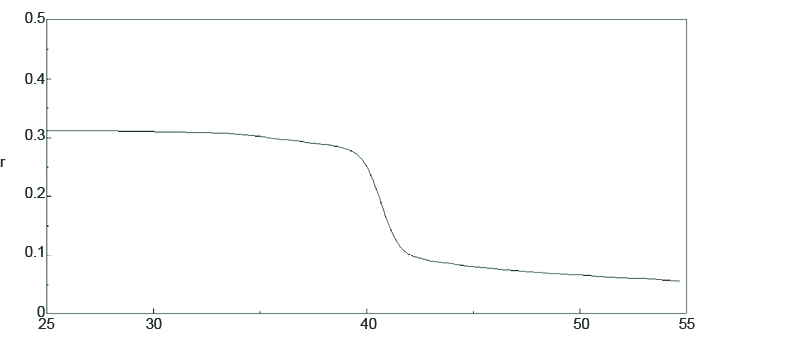
Figure 1 shows the temperature-dependent fluorescence anisotropy of 0.6 µM of DPH in 130 µM of phosphatide. The spectrum indicates that DPH is oriented between the lipid bilayer and is restricted from movement at a low temperatures. The phase transition of the membrane at 40°C reorients the membrane, allowing DPH to move freely and decreasing the polarization.
Conclusion
This application note demonstrates that the FP-6500 can be used with automatic polarizers to obtain information regarding the mobility of fluorophores under different environmental conditions.
Required Products and Software
- FP-8300/8500/8600/8700 Spectrofluorometer
- FDP-837 Automatic Polarizer
- ETC-813 Air-cooled Peltier Thermostatted Cell Holder with Stirrer, ETC-814/815 Water-cooled Peltier Thermostatted Cell Holder with Stirrer, or PTC-818 Water-cooled Peltier Thermostatted 4-position Automatic Cell Changer with Stirrer
- FWAP-875 Fluorescence Polarization Measurement program
- FWTP-874 Temperature Control Measurement program
Featured Products:
-

A powerful combination of performance, sensitivity and flexibility for biological, environmental and materials analysis.
FP-8350 Spectrofluorometer
-

Sophisticated optical system offering the ultimate in sensitivity, spectral accuracy, and flexibility for the most challenging materials and biological samples.
FP-8550 Spectrofluorometer
-
FP-8650 NIR Spectrofluorometer

Fluorescence Depolarization Measurement of Liposome
Introduction

Fluorescence depolarization or fluorescence anisotropy can be observed when a fluorophore emits light of different intensities depending on the axes of polarization. When a molecule is excited by the absorption of light from its ground state to a transition state and relaxes to a stable vibrational state, it will then return back to its ground state by radiating fluorescence light. In this process there is a directionality, described by a transition dipole moment and determined by molecular structure, which is closely related to the direction of polarization of the excitation and fluorescence light.
For example, when fluorescence is emitted before a molecule rotates, the fluorescence light will be strongly polarized towards the direction of the excitation light’s polarization. If the light is emitted after the rotation of the molecule in a completely random direction, the fluorescence will be no longer polarized.
When measuring fluorescence polarization, the following factors will affect the molecular movement: (1) molecular size, (2) viscosity of the molecule’s environment, and (3) strength and degrees of freedom of a bound molecule. Larger molecules and more viscous or populated conditions require more energy in order for a molecule to move.
This application note will demonstrate the use of the FP-6500 spectrofluorometer and its automatic polarizers to monitor the temperature-dependent polarization of diphenyl hexatriene (DPH) upon addition to a lipid bilayer.
Measurement Conditions
| Excitation Wavelength | 357 nm | Emission Wavelength | 430 nm |
| Excitation Bandwidth | 3 nm | Emission Bandwidth | 3 nm |
| Response Time | 2 sec | Data Interval | 0.1 °C |
| Temperature Gradient | 20°C/hr | Measurement Mode | Fluorescence |
Experimental
The fluorescence polarization spectrum can be measured using the automatic polarizers. By placing the excitation polarizer in the vertical position and the emission polarizer in the horizontal and vertical positions, both fluorescence intensities, IVH and IVV, are measured, respectively. In order to correct for the sensitivity difference of the emission detector, the spectrum of rhodamine B is measured by setting the excitation polarizer in the horizontal position and setting the emission polarizer in the vertical and horizontal positions. The instrument grating factor is then defined as the following:
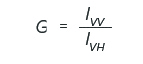
and multiplied by IVH to obtain an instrumental correction factor for the emission optics. The degree of polarization (P) is given by the following equation:
![]()
Results
Figure 1 shows the temperature-dependent fluorescence anisotropy of 0.6 µM of DPH in 130 µM of phosphatide. The spectrum indicates that DPH is oriented between the lipid bilayer and is restricted from movement at a low temperatures. The phase transition of the membrane at 40°C reorients the membrane, allowing DPH to move freely and decreasing the polarization.

Conclusion
This application note demonstrates that the FP-6500 can be used with automatic polarizers to obtain information regarding the mobility of fluorophores under different environmental conditions.
Keywords
210-FP-0016, FP-6500, Fluorescence, Polarization, Biochemistry
Required Products and Software
- FP-8300/8500/8600/8700 Spectrofluorometer
- FDP-837 Automatic Polarizer
- ETC-813 Air-cooled Peltier Thermostatted Cell Holder with Stirrer, ETC-814/815 Water-cooled Peltier Thermostatted Cell Holder with Stirrer, or PTC-818 Water-cooled Peltier Thermostatted 4-position Automatic Cell Changer with Stirrer
- FWAP-875 Fluorescence Polarization Measurement program
- FWTP-874 Temperature Control Measurement program

 Download This Application
Download This Application