Quantum Efficiency of Phosphorescent Materials using an Integrating Sphere
May 1, 2024
Introduction

Phosphorescent compounds are frequently used as the luminescent layer in organic electro-luminescent devices, this is because the radiative decay from both the triplet and singlet states of a phosphorescent material increases its quantum efficiency, compared to materials where only a singlet state contributes to light emission. These phosphorescent materials must have a certain level of quantum efficiency for practical use. Quantum efficiency is defined as the ratio of the number of photons absorbed by sample to the number of photons emitted by sample
To measure phosphorescent materials in materials such as films, an integrating sphere is required. An integrating sphere collects the light that is scattered from a surface in different directions and focuses that light to a detector, as shown in Figure 1. While conventional integrating spheres typically only measure sample spectra at room temperature, the phosphorescence of a wide range of samples can be observed if the relaxation process is slowed down by cooling samples to 77 Kelvin.
This application note evaluates a system developed for calculating quantum efficiency from phosphorescence spectra measured at 77 Kelvin.
Experimental
To obtain phosphorescence data with an integrating sphere a Dewar vessel, filled with coolant such as liquid nitrogen, is placed in the sphere and the spectrum of the incident light is measured as illustrated in Figure 1.
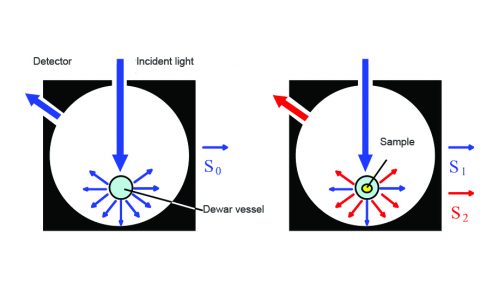
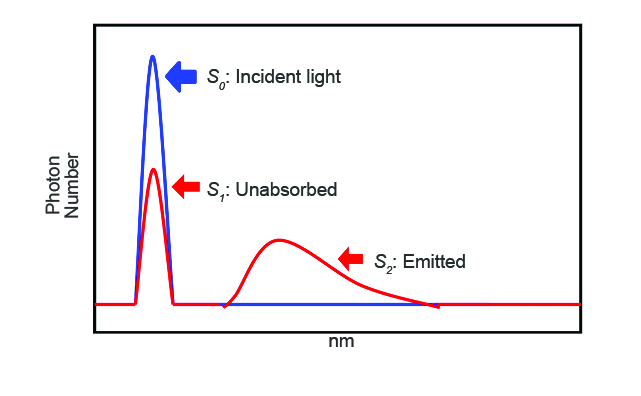
The incident photon (S0) peak area appears in the excitation spectrum, illustrated in blue in Figure 2. The sample is then placed inside the Dewar vessel in the integrating sphere (Figure 1, right) and the sample’s excitation and emission spectra are measured. Figure 2 shows the excitation and emission of the scattering incident light. The excitation and emission peaks indicate the number of photons unabsorbed (S1) and emitted (S2) by the sample, respectively. The quantum efficiency is calculated using the following equation:
![]()
Measurement Conditions
| Excitation Bandwidth | 5 nm | Excitation Wavelength | 350 (Quinine Sulfate), 335 (Benzophenone) nm |
| Emission Bandwidth | 5 nm | Data Interval | 1 nm |
| Response Time | 0.5 sec | Scanning Speed | 1000 nm/min |
Keywords
190-FP-0006, FP-6500, FWSQ-6017, Fluorescence, Phosphorescence, Quantum Efficiency, Materials, Integrating Sphere
Results
To confirm whether placing the Dewar vessel1 and coolant in the integrating sphere has any effect on the measurement results, the fluorescence spectrum of a sample with a known quantum efficiency was measured. Figure 3 illustrates the measured fluorescence spectrum and Table 1 shows the calculated quantum efficiencies (Φ). The calculated fluorescence quantum efficiency was 0.56 while the literature value was 0.5462. These results indicate no substantial effects when placing a dewar inside the integrating sphere.
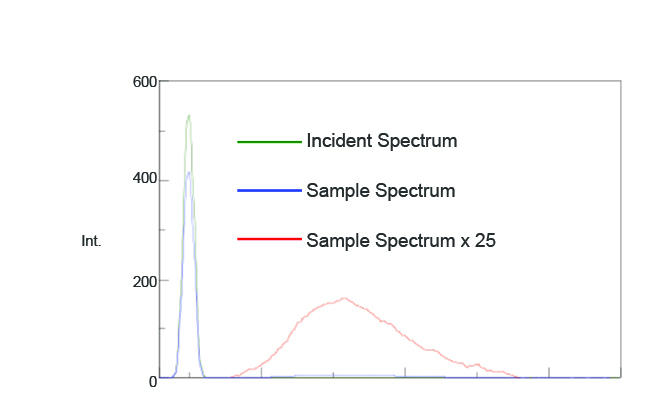
Table 1. Fluorescence intensity and quantum efficiency values of quinine sulfate.
| Intensity | |
| S0 | 4954.3 |
| S1 | 4074.0 |
| S2 | 819.3 |
| ΦMeasured | 0.93 |
| ΦLiterature3 | 0.90 |
A sample of benzophenone was cooled by liquid nitrogen and placed in the integrating sphere. Figure 4 illustrates the measured fluorescence spectrum and Table 2 shows the calculated quantum efficiencies (Φ). The calculated phosphorescence quantum efficiency was 0.93 while the literature value was 0.903.
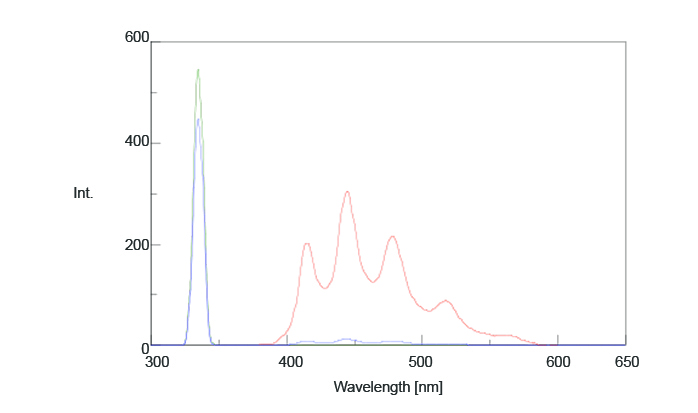
Table 2. Fluorescence intensity and quantum efficiency values of benzophenone.
| Intensity | |
| S0 | 4954.3 |
| S1 | 4074.0 |
| S2 | 819.3 |
| ΦMeasured | 0.93 |
| ΦLiterature3 | 0.90 |
Conclusion
The quantum efficiency of phosphorescent materials at low temperatures can reliably be obtained using an FP-6500 and a dedicated quantum efficiency system.
Required Products and Software
- FP-8300/8500/8600/8700 Spectrofluorometer
- ILFC-847 LN2 Cooled 100 mm dia. Integrating Sphere
- PMU-830 Liquid Nitrogen Cooling Unit
- PPH-150 Phosphorescence Measurement Cell Kit for Powder Sample
- FWQE-880 Quantum Yield Cal culation program
References
1. In this measurement, the Dewar vessel was filled with water instead of liquid nitrogen
2. Melhuish, W.H., Journal of Physical Chemistry. 65, 229, 1961.
3. The Chemical Society of Japan, Courses in Experimental Chemistry 3 Basic Physical Chemistry.
Featured Products:
-

A powerful combination of performance, sensitivity and flexibility for biological, environmental and materials analysis.
FP-8350 Spectrofluorometer
-

Sophisticated optical system offering the ultimate in sensitivity, spectral accuracy, and flexibility for the most challenging materials and biological samples.
FP-8550 Spectrofluorometer
-
FP-8650 NIR Spectrofluorometer

Quantum Efficiency of Phosphorescent Materials using an Integrating Sphere
Introduction

Phosphorescent compounds are frequently used as the luminescent layer in organic electro-luminescent devices, this is because the radiative decay from both the triplet and singlet states of a phosphorescent material increases its quantum efficiency, compared to materials where only a singlet state contributes to light emission. These phosphorescent materials must have a certain level of quantum efficiency for practical use. Quantum efficiency is defined as the ratio of the number of photons absorbed by sample to the number of photons emitted by sample
To measure phosphorescent materials in materials such as films, an integrating sphere is required. An integrating sphere collects the light that is scattered from a surface in different directions and focuses that light to a detector, as shown in Figure 1. While conventional integrating spheres typically only measure sample spectra at room temperature, the phosphorescence of a wide range of samples can be observed if the relaxation process is slowed down by cooling samples to 77 Kelvin.
This application note evaluates a system developed for calculating quantum efficiency from phosphorescence spectra measured at 77 Kelvin.
Experimental
To obtain phosphorescence data with an integrating sphere a Dewar vessel, filled with coolant such as liquid nitrogen, is placed in the sphere and the spectrum of the incident light is measured as illustrated in Figure 1.

The incident photon (S0) peak area appears in the excitation spectrum, illustrated in blue in Figure 2. The sample is then placed inside the Dewar vessel in the integrating sphere (Figure 1, right) and the sample’s excitation and emission spectra are measured. Figure 2 shows the excitation and emission of the scattering incident light. The excitation and emission peaks indicate the number of photons unabsorbed (S1) and emitted (S2) by the sample, respectively. The quantum efficiency is calculated using the following equation:
![]()

Measurement Conditions
| Excitation Bandwidth | 5 nm | Excitation Wavelength | 350 (Quinine Sulfate), 335 (Benzophenone) nm |
| Emission Bandwidth | 5 nm | Data Interval | 1 nm |
| Response Time | 0.5 sec | Scanning Speed | 1000 nm/min |
Results
To confirm whether placing the Dewar vessel1 and coolant in the integrating sphere has any effect on the measurement results, the fluorescence spectrum of a sample with a known quantum efficiency was measured. Figure 3 illustrates the measured fluorescence spectrum and Table 1 shows the calculated quantum efficiencies (Φ). The calculated fluorescence quantum efficiency was 0.56 while the literature value was 0.5462. These results indicate no substantial effects when placing a dewar inside the integrating sphere.

Table 1. Fluorescence intensity and quantum efficiency values of quinine sulfate.
| Intensity | |
| S0 | 4954.3 |
| S1 | 4074.0 |
| S2 | 819.3 |
| ΦMeasured | 0.93 |
| ΦLiterature3 | 0.90 |
A sample of benzophenone was cooled by liquid nitrogen and placed in the integrating sphere. Figure 4 illustrates the measured fluorescence spectrum and Table 2 shows the calculated quantum efficiencies (Φ). The calculated phosphorescence quantum efficiency was 0.93 while the literature value was 0.903.

Table 2. Fluorescence intensity and quantum efficiency values of benzophenone.
| Intensity | |
| S0 | 4954.3 |
| S1 | 4074.0 |
| S2 | 819.3 |
| ΦMeasured | 0.93 |
| ΦLiterature3 | 0.90 |
Conclusion
The quantum efficiency of phosphorescent materials at low temperatures can reliably be obtained using an FP-6500 and a dedicated quantum efficiency system.
Keywords
190-FP-0006, FP-6500, FWSQ-6017, Fluorescence, Phosphorescence, Quantum Efficiency, Materials, Integrating Sphere
Required Products and Software
- FP-8300/8500/8600/8700 Spectrofluorometer
- ILFC-847 LN2 Cooled 100 mm dia. Integrating Sphere
- PMU-830 Liquid Nitrogen Cooling Unit
- PPH-150 Phosphorescence Measurement Cell Kit for Powder Sample
- FWQE-880 Quantum Yield Cal culation program
References
1. In this measurement, the Dewar vessel was filled with water instead of liquid nitrogen
2. Melhuish, W.H., Journal of Physical Chemistry. 65, 229, 1961.
3. The Chemical Society of Japan, Courses in Experimental Chemistry 3 Basic Physical Chemistry.

 Download This Application
Download This Application