What is High-Performance Liquid Chromatography (HPLC)?
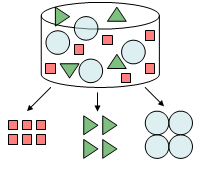
High-Performance Liquid Chromatography (HPLC) is an analytical technique used to separate, identify, and quantify components in a mixture. Chromatography refers to the measurement method, chromatogram refers to the measurement results, and chromatograph refers to the instrument. Chromatography can be used for qualitative or quantitative analysis. Qualitative analysis refers to “what components are in the mixture” while quantitative analysis refers to “how much of each component is present in the mixture” (Fig. 1).
The Beginning of Chromatography
Methods for separating components of a mixture include filtration, distillation, and extraction. Chromatography was invented by the Russian botanist Mikhail Semenovich Tswett. In the early 1900s, Tswett packed calcium carbonate in a standing tube, placed pigments extracted from plants on top, and then flushed the tube with petroleum ether as a solvent (Fig. 2).
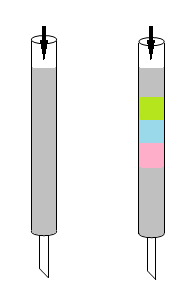
As the extracted pigments began to separate on the chromatograph, each individual pigment appeared as a different band of color as if the light was divided into seven colors by a prism in the tube. For this reason, Tswett named this new separation method chromatography using the Greek words chroma, which means color, and graph, which means record. HPLC is a type of separation method commonly referred to as column chromatography and has been developed to enable separation and analysis in a short period of time by increasing pressure compared to atmospheric pressure.
Separation Mechanism in Chromatography
A mixture is placed in a stream of liquid (petroleum ether in Fig. 3) called the mobile phase and moved through a solid medium (calcium carbonate powder in Fig. 3) called the stationary phase. The components in the mixture move with the flow of the mobile phase and interact with the stationary phase. The speed of movement depends on the strength of the interaction between each component and the stationary phase. That is, components that interact strongly with the stationary phase move slowly, whereas components that interact weakly move quickly, so allowing the components to be separated.
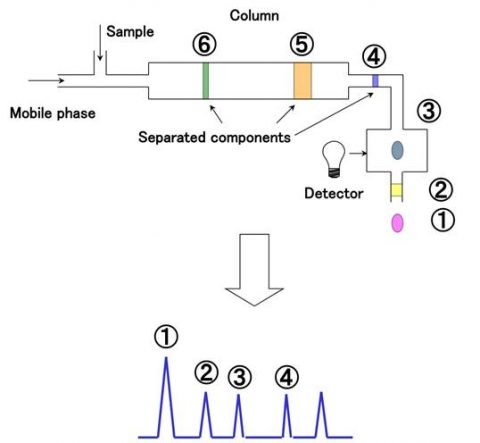
The separated components can be analyzed using different types of detectors. A UV detector, for example, can detect components based on UV absorption. The chromatogram is obtained by measuring the elution time on the X axis and the intensity of the UV signal on the Y-axis. If the measurement conditions are the same, the elution time (peak position) for the standard sample whose components are known and that for the unknown sample can be compared to identify the components for qualitative analysis. In addition, since the absorption intensity is proportional to the concentration, a calibration curve can be prepared using a standard sample, and the component concentration can be determined by measuring the peak area or height for quantitative analysis.
