Separation Principle
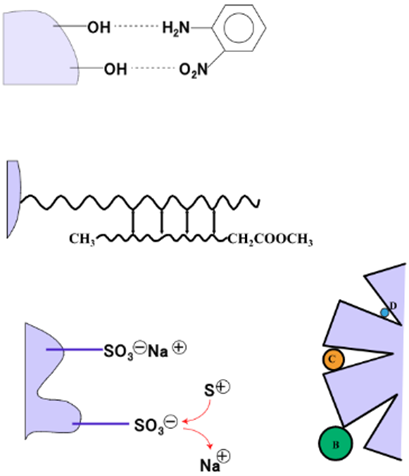
In High-Performance Liquid Chromatography (HPLC), individual components of a mixture are separated using a column based on the difference in the degree of interaction between the sample components and the stationary phase of the column. Components with a low degree of interaction with the stationary phase are eluted first. These interactions include adsorption, hydrophilic and hydrophobic interactions, electron affinity, penetration, and exclusion (Fig. 1).
- Column Types and Separation Modes
- Normal Phase vs. Reverse Phase
- Isocratic Elution vs. Gradient Elution
Column Types and Separation Modes
As shown in the table below, there are various types of columns and separation modes that can be used, and the optimum choice depends on the nature of the sample and the analysis that is required. When an organic solvent is used as the mobile phase, a normal-phase column (mainly silica gel) can separate and analyze samples composed of fat-soluble components based on adsorption. When a water/methanol solvent composition is used as the mobile phase, a reverse-phase column (mainly carbon chains bonded to silica) can separate and analyze samples based on hydrophobic interactions. Gel Permeation Chromatography (GPC) and Gel Filtration Chromatography (GFC) columns both separate sample components based on their molecular size. The difference between the two is that GPC uses an organic solvent as a mobile phase while GFC uses an aqueous solution as the mobile phase. Finally, ion exchange columns separate and analyze samples composed of ionic components based on the electrical affinity.
| Column Type | Stationary Phase | Mobile Phase | Interaction | Features |
|---|---|---|---|---|
| Normal phase | Silica gel | Organic solvent | Adsorption | Separation of fat-soluble components |
| Reversed phase | Silica C18 (ODS) | Water / MeOH | Hydrophobic | The most common method |
| GPC (Non-aqueous) | Polymer | Organic solvent | Gel permeation | Molecular weight distribution measurement |
| GFC (Aqueous) | Hydrophilic polymer | Buffer | Gel permeation | Biopolymer separation |
| Ion exchange | Ion exchanger | Buffer | Electric affinity | Separation of ionic components |
Normal Phase vs. Reverse Phase
Normal-phase chromatography and reverse-phase chromatography are completely different separation methods. In normal-phase chromatography, a low-polarity solvent is passed through a high-polarity column, resulting in the low-polarity components being eluted first. In reverse-phase chromatography, a high-polarity solvent is passed through a non-polar column, resulting in the high-polarity components being eluted first.
Isocratic Elution vs. Gradient Elution
Isocratic elution occurs when the composition of the mobile phase remains constant throughout the course of the separation. Gradient elution occurs when the composition of the mobile phase changes over the course of the separation.
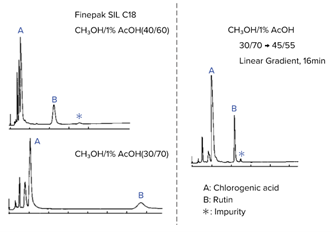
In reverse-phase chromatography and ion-exchange chromatography, gradient elution can be used to improve separation and reduce measurement time. For example, chlorogenic acid and rutin can be separated using an ODS column (Finepak SIL C18) and a methanol/1% acetic acid solution (Fig. 2).
First, let’s look at an isocratic elution of a methanol/1% acetic acid ratio of 40/60 (Fig. 2, upper left). In this example, component A could not be separated well; however, when the ratio was changed from 40/60 to 30/70 (Fig. 2, bottom left), separation was achieved, but it took a long time. By applying a gradient from a methanol/1% acetic acid ratio of 30/70 to 45/55 (changing the composition of the mobile phase over the course of the separation), the retention of the column was strengthened, resulting in the successful separation and elution of component A (Fig. 2, right).