How does ATR FTIR?
There are various FTIR methods that can be used to identify foreign materials or contaminants. FTIR microscopy is used for foreign material where the size is in the order of a few microns, but when a sample with foreign material can be observed by the naked eye, the particle type is typically greater than several hundred microns and does not require an FTIR microscope. An ATR FTIR method may be a suitable alternative and offers advantages such as minimal sample preparation, non-destructive measurement, and easy handling.
The use of Attenuated Total Reflectance (ATR) in FTIR spectroscopy has become the primary sampling method for FTIR spectroscopy. The major advantage is the lack of sample preparation for liquid and solid samples. When light reflects off of certain materials (diamond, ZnSe, etc.) at a critical angle, the light undergoes total internal reflectance with the propagation of an evanescent field from the surface of the crystal that interacts with the material (sample) in contact, Figure 1. The penetration depth is dependent on the refractive index of both the sample (generally ~1.5) and the crystal itself. Since the refractive index is dependant on wavelength, spectra taken with ATR have slightly different intensity ratios across the spectrum and may need to be corrected to compare to transmission spectra.
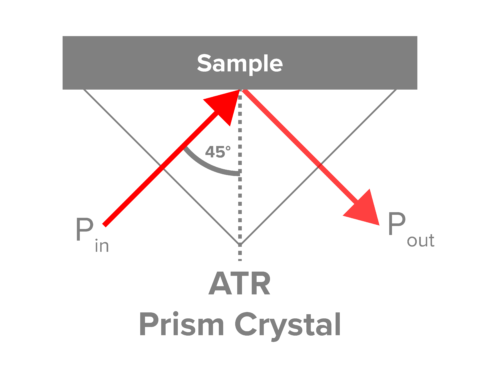
The three most common crystals are diamond, zinc selenide, and germanium, each having advantages and disadvantages. Diamond crystals are rugged, have a penetration depth of 1.5 μm, low wavenumber cutoff (200 cm-1), but have poor throughput in the 2,200 cm-1 region. ZnSe has exceptional throughput but a high cutoff (650 cm-1). Germanium has a very low penetration depth (0.8 μm) and is useful for highly absorbing substances. The properties and care of common ATR crystals can be found in Table 1.
Types of ATR Prism Crystals
ATR method is used to analyze the sample surface within 1~2 μm in depth. The measurement is collected when the ATR prism is in contact with the sample. In the ATR method, the penetration depth of light into the sample for total reflection depends on the refractive index of the prism and sample, incidence angle, and wavelength. In addition, measurement range and durability depend on the material of the prism. Features of each prism are shown as follows.
| Prism | RI (n1) | Penetration Depth dp*1 (1000cm-1) | RI of Sample (n2)*2 for Total Reflection | Measurement Limit in Low Wavenumber*3 Range |
| ZnSe | 2.4 | ~ 2.0 μm | <= 1.7 | ~550 cm-1 |
| Diamond | 2.4 | ~ 2.0 μm | <= 1.7 | ~400 cm-1 *4 |
| Ge | 4.0 | ~ 2.0 μm | <= 2.8 | ~650 cm-1 |
| Prism | Feature of Prism | Suitable Sample | Non-Suitable Sample | Notes |
| ZnSe | Good throughput | General organic substance | Hard powder, acidalkaline, high RI sample | In case of hard powder or hubbly sample, diamond is recommended |
| Diamond | Intensity, durability, measurement | Hard powder, General organic substance | High RI sample | Poor S/N ratio in the region around 2000 cm-1, due to internal absorption |
| Ge | Analysis for high RI sample | Sample including carbon | Hard powder, acidalkaline | Weak absorption due to small depth of penetration |
