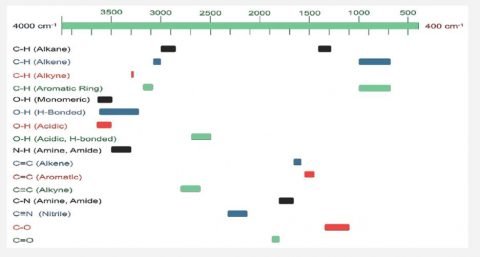
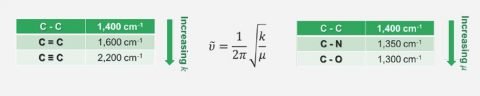FTIR
Complementary Techniques:
-
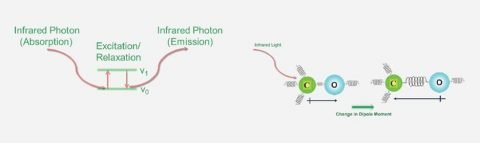
FTIR microscopy is ideal for imaging materials using functional group(s), sample identification, multilayer film characterization, and particle analysis.
FTIR Microscopy
-
This technique can be used for chemical or molecular analysis encompassing depth profiling and mapping of samples with spatial resolution as little as 1 μm.
Confocal Raman Microscopy
-
An FTIR ATR method may be a suitable alternative and offers advantages such as minimal sample preparation, non-destructive measurement, and easy handling.
ATR FTIR